Methods of treating tumors
a tumor and tumor technology, applied in the field of tumor treatment methods, can solve problems such as negative impact on the patient's experien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ous Injection Formulation Development
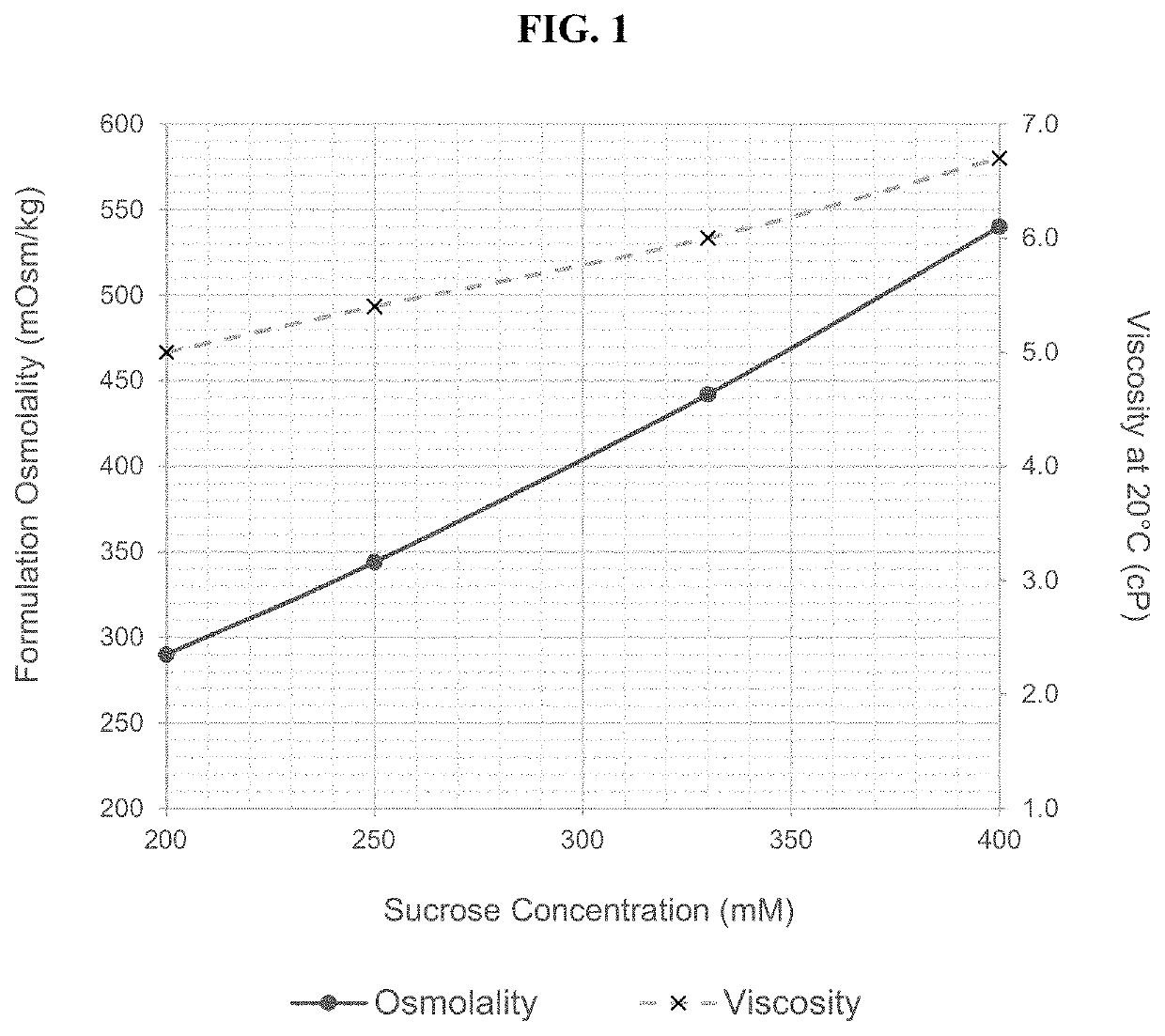
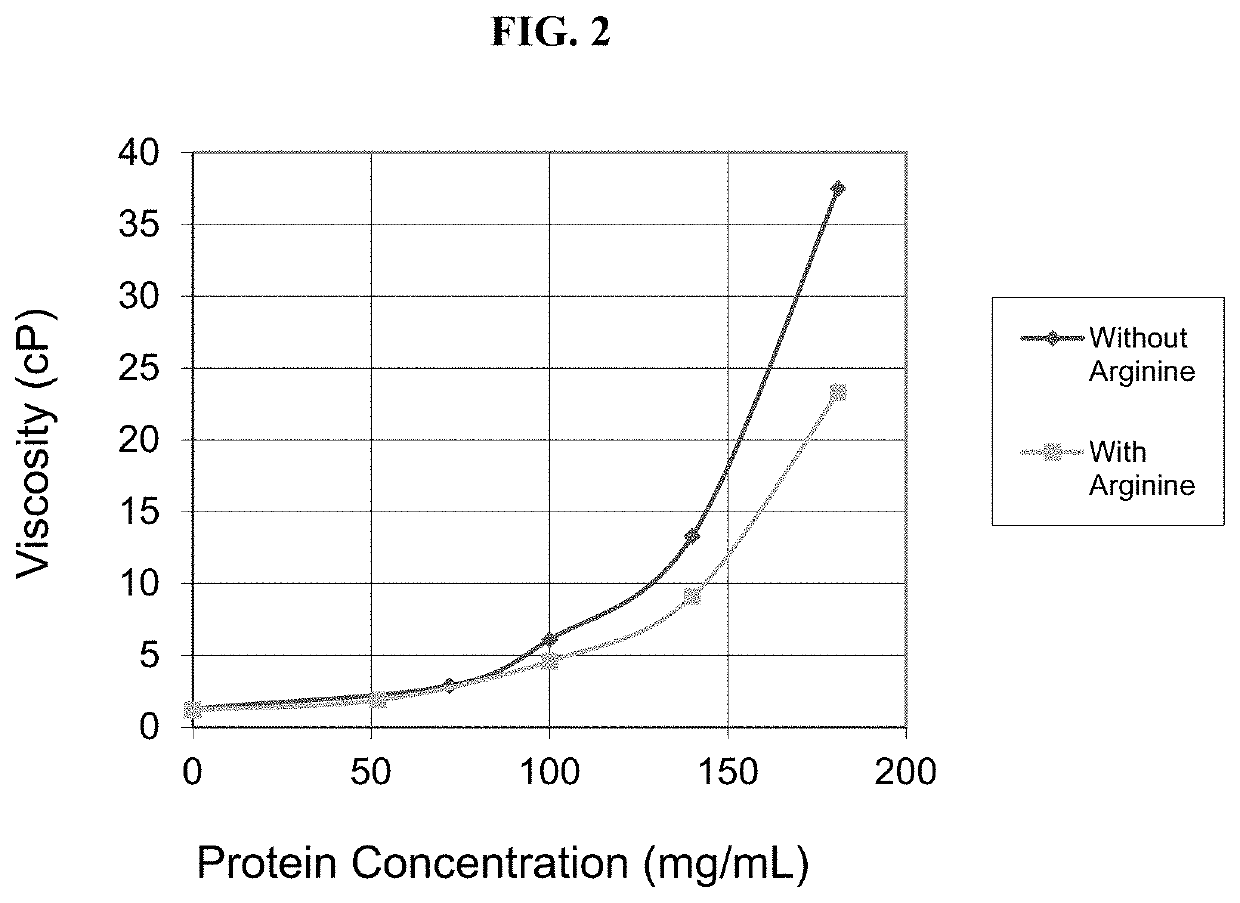
[0461]The present example discusses the development of a stable, robust subcutaneous (SC) formulation of nivolumab and a manufacturing process suitable for commercial scale production. As a part of the formulation studies, the effects of various different pharmaceutically acceptable excipients on the stability of nivolumab were evaluated. Studies were also undertaken to select processing and packaging components compatible with the selected formulation. In addition, use time studies were conducted to support administration of the drug product via subcutaneous injection.
[0462]The objectives of these studies conducted for the development of nivolumab SC injection include: 1. identification and development of a stable injectable formulation for nivolumab SC injection that would be suitable for clinical use and eventual commercialization; 2. identification of manufacturing equipment and packaging components that are compatible with nivolumab SC injec...
example 2
ous Nivolumab with or without rHuPH20
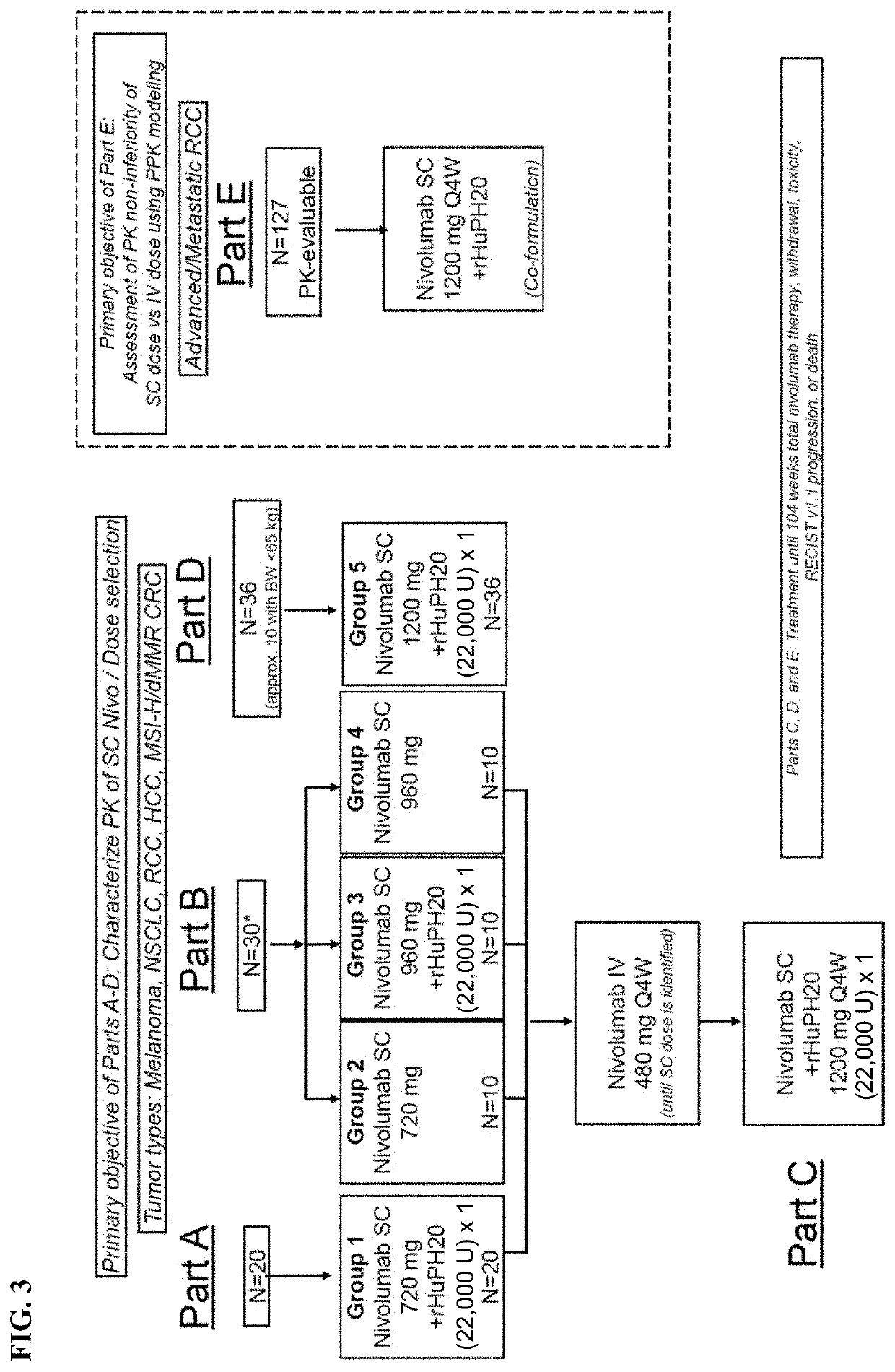
[0577]In an ongoing Phase 1 / 2 study the PK, safety, efficacy, and tolerability was evaluated for nivolumab monotherapy administered subcutaneously (SC) with or without the hyaluronidase rHuPH20 in patients across solid tumors (metastatic melanoma, RCC, NSCLC, HCC, and CRC) where PK, efficacy, safety, and immunogenicity of nivolumab following IV administration have been well-characterized. Other solid tumors where PK of IV nivolumab was well-characterized (gastroesophageal junction [GEJ], gastric cancer (GC), metastatic urothelial carcinoma (mUC) and SCCHN were permitted).
[0578]The starting SC dose selected for Part A was 720 mg Q4W. Based on preliminary PK from Part A and subsequent modeling, this study proceeded as planned with a second dose of 960 mg Q4W for Part B.
[0579]For Parts A and B, PK of single dose SC nivolumab (with and without rHuPH20) was characterized, followed by IV nivolumab 480 mg Q4W at Week 4. These SC PK data were used to upd...
example 3
ous Nivolumab in Combination with Recombinant Human Hyaluronidase in Previously Treated Advanced, Recurrent, or Metastatic Non-Small Cell Lung Cancer
[0616]A phase 3 study will be performed to evaluate the administration of subcutaneous (SC) nivolumab coformulated with recombinant human PH20 (rHuPH20) versus intravenous (IV) nivolumab in participants with previously treated advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC). This study seeks to establish pharmacokinetic (PK) and efficacy non-inferiority of 1200 mg of nivolumab coformulated with 20,000 Units of rHuPH20 administered SC every 4 weeks (Q4W) compared with 3 mg / kg nivolumab administered IV every 2 weeks (Q2W). Throughout the protocol, the coformulation of nivolumab and rHuPH20 will be referred to as SC nivolumab and the IV formulation of nivolumab will be referred to as IV nivolumab.
[0617]Males or females 18 years of age or older (or local age of majority) with histologically confirmed Stage IIIB / IIIC / IV...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com