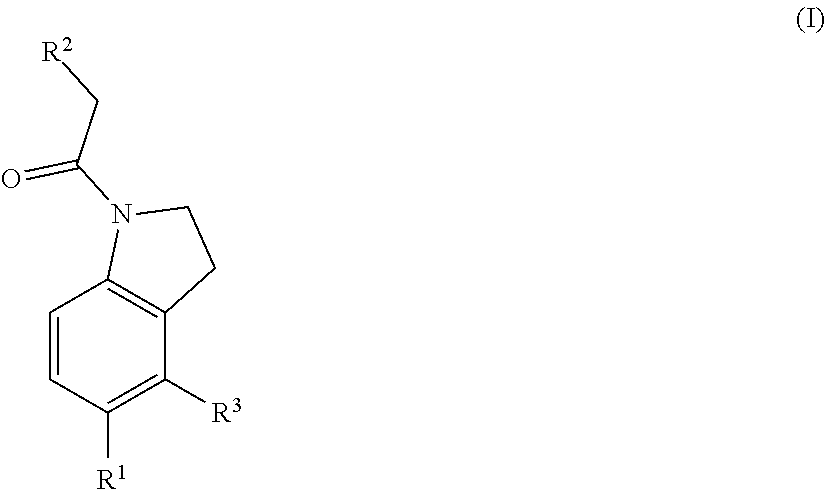
Chemical compounds
a technology of indoline and derivatives, applied in the field of substituted indoline derivatives, can solve the problems of reducing the rate of translation initiation and triggering cell death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-methyl-3-[1-(phenylacetyl)-2,3-dihydro-1H-indol-5-yl]-1H-pyrazolo[3,4-d]pyrimidin-4-amine
[0529]
5-bromo-1-(phenylacetyl)-2,3-dihydro-1H-indole
[0530]To a mixture of phenylacetic acid (0.687 g, 5.05 mmol) and HATU (2.112 g, 5.55 mmol) in N,N-Dimethylformamide (DMF) (5 mL) was added Hunig's base (0.882 mL, 5.05 mmol), and the resulting mixture was stirred for 15 minutes at room temperature. 5-bromo-2,3-dihydro-1H-indole (1 g, 5.05 mmol) was added, and the reaction mixture was stirred at room temperature overnight. The reaction was poured onto water, and the resulting precipitate was filtered and air dried to afford the 5-bromo-1-(phenylacetyl)-2,3-dihydro-1 H-indole (1.24 g) as a tan solid.
1-methyl-3-[1-(phenylacetyl)-2,3-dihydro-1H-indol-5-yl]-1H-pyrazolo[3,4-d]pyrimidin-4-amine
[0531]To 5-bromo-1-(phenylacetyl)-2,3-dihydro-1H-indole (122 mg, 0.386 mmol), bis(pinacolato)diboron (125 mg, 0.491 mmol), PdCl2(dppf)-CH2Cl2 adduct (28.6 mg, 0.035 mmol) were added 1,4-Dioxane (2 mL) and ammo...
example 2
3-{1-[(2,5-difluorophenyl)acetyl]-2,3-dihydro-1H-indol-5-yl}-1-methyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine
[0532]
5-bromo-1-[(2,5-difluorophenyl)acetyl]-2,3-dihydro-1H-indole
[0533]To a mixture of (2,5-difluorophenyl)acetic acid (0.869 g, 5.05 mmol) and HATU (2.112 g, 5.55 mmol) in N,N-Dimethylformamide (DMF) (10 mL) was added Hunig's base (0.882 mL, 5.05 mmol), and the resulting mixture was stirred for 15 minutes at room temperature. 5-bromo-2,3-dihydro-1H-indole (1 g, 5.05 mmol) was added, and the reaction mixture was stirred at room temperature for 1 hour. The mixture was poured onto water, and the resulting aqueous mixture was filtered to afford 5-bromo-1-[(2,5-difluorophenyl)acetyl]-2,3-dihydro-1H-indole (1.6 g) as a tan solid.
3-{1-[(2,5-difluorophenyl)acetyl]-2,3-dihydro-1H-indol-5-yl}-1-methyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine
[0534]To a mixture of 5-bromo-1-[(2,5-difluorophenyl)acetyl]-2,3-dihydro-1H-indole (160 mg, 0.454 mmol), bis(pinacolato)diboron (127 mg, 0.500 mmol), and ...
example 3
3-[1-(phenylacetyl)-2,3-dihydro-1H-indol-5-yl]-1H-pyrazolo[3,4-d]pyrimidin-4-amine
[0535]
3-[1-(phenylacetyl)-2,3-dihydro-1H-indol-5-yl]-1H-pyrazolo[3,4-d]pyrimidin-4-amine
[0536]To a mixture of 5-bromo-1-(phenylacetyl)-2,3-dihydro-1H-indole (148 mg, 0.467 mmol),bis(pinacolato)diboron (125 mg, 0.491 mmol), and potassium acetate (138 mg, 1.402 mmol) was added 1,4-dioxane (6 mL), and the mixture was degassed with N2 for 10 minutes. PdCl2(dppf)-CH2Cl2 adduct (19.08 mg, 0.023 mmol) was added, and the reaction mixture was stirred for 3 hours at 100 C into a sealed vessel. The reaction was cooled down to room temperature. 3-bromo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (100 mg, 0.467 mmol) and sat. aq. NaHCO3 (2 mL) were added, and N2 gas was bubbled through the mixture for 10 minutes. PdCl2(dppf)-CH2Cl2 adduct (19.08 mg, 0.023 mmol) was added, the vessel was sealed, and the reaction mixture was stirred for 3 days at 100 C. The mixture was allowed to cool to room temperature and poured onto wate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com