Methods of treating fragile X associated disorders, ADHD, and autism spectrum disorder
a technology of adhd and autism spectrum disorder, applied in the direction of anhydride/acid/halide active ingredients, drug compositions, tetracycline active ingredients, etc., can solve the problems of early cessation, high and increased risk of abuse of stimulants
Active Publication Date: 2017-12-12
OTSUKA AMERICA PHARMA INC
View PDF21 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
This approach provides therapeutic benefits by enhancing dopamine function without the pronounced dopaminergic activities of stimulants, reducing the risk of substance abuse and off-target interactions, thereby improving cognitive, behavioral, and emotional symptoms in patients with fragile X-associated disorders.
Problems solved by technology
Affected women may exhibit intermittent and unpredictable menses, with eventual early cessation.
However, concerns about stimulants include risk of abuse, dependency, and diversion as well as potential neurotoxic effects of amphetamines.
It has been reported, however, that some individuals with FXS failed treatment predominately due to aggravation of aggressive, perseverative, and irritable behavior.
Six trials of atomoxetine in individuals with FXS, however, were unsuccessful, with the majority failing due to aggravation of irritable, moody, and aggressive behaviors.
However, as discussed above, concerns about treatment with stimulants include risk of abuse, dependency, and diversion as well as potential neurotoxic effects of amphetamines.
In addition, stimulants may exacerbate anxiety.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example
Example 1
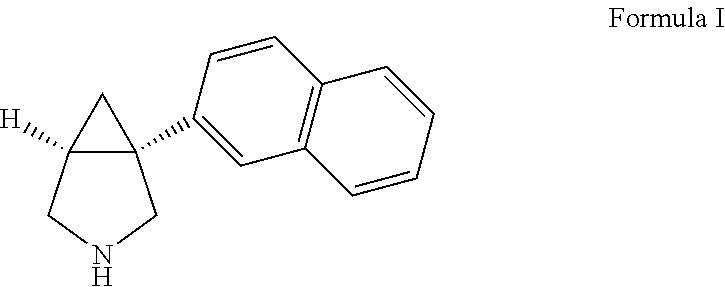
[0325]Sustained release pharmaceutical composition comprising (1R,5S)-1-(naphthalen-2-yl)-3-azabicyclo[3.1.0]hexane hydrochloride
[0326]
Tablet UnitConcentrationWeightIngredient(% W / W)(mg)(1R,5S)-1-(naphthalen-2- 25%100yl)-3-azabicyclo[3.1.0]hexanehydrochlorideLactose, Monohydrate,74.5% 298NFHypromellose, NF(as 50 / 50 premix -RetaLac ®)Magnesium Stearate, NF 0.5%2(Hyqual ® Vegetablesource)Total100%400
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| spectrum disorder | aaaaa | aaaaa |
| fragile | aaaaa | aaaaa |
| weight/weight | aaaaa | aaaaa |
Login to View More
Abstract
Provided are novel methods comprising administering a therapeutically effective amount of (1R,5S)-1-(naphthalen-2-yl)-3-azabicyclo[3.1.0]hexane, in free or pharmaceutically acceptable salt form, to a patient in need thereof.
Description
CROSS REFERENCE TO RELATED APPLICATIONS[0001]This application is the National Stage Entry under 35 U.S.C. §371 of International Application No. PCT / US2014 / 069401 filed Dec. 9, 2014, which claims priority to U.S. Provisional Application No. 61 / 913,886 filed Dec. 9, 2013, the contents of both of which are hereby incorporated by reference.BACKGROUND[0002]Fragile X-associated disorders are a family of genetic conditions that may affect individuals in a variety of ways. The three fragile X-associated disorders are fragile X syndrome (FXS), fragile X-associated tremor / ataxia syndrome (FXTAS), and fragile X-associated primary ovarian insufficiency (FXPOI). The conditions are all caused by changes in the fragile X mental retardation 1 (FMR1) gene, located on the X chromosome. In most people, the FMR1 gene contains approximately 5-44 repeats of the cytosine guanine guanine (CGG) trinucleotide within the 5′ untranslated region.[0003]Fragile X syndrome (FXS) is the most common inherited form o...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(United States)
IPC IPC(8): A61K31/403A61K31/185A61K31/27A61K31/65A61K9/20A61K45/06
CPCA61K31/403A61K9/2013A61K45/06A61K31/65A61K31/27A61K31/185A61K9/2054A61K9/2018A61K2300/00A61P25/28A61P25/30A61P43/00
Inventor MCKINNEY, ANTHONYGENTILE, FRANKHSU, TIMOTHYBYMASTER, FRANKLINPISKORSKI, WALTERWELTER, RICHARD
Owner OTSUKA AMERICA PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com