Semicarbazones having CNS activity and pharmaceutical preparations containing same
a technology of central nervous system and semicarbazone, which is applied in the direction of biocide, animal repellents, drug compositions, etc., can solve the problems of low protection index, namely the ratio td.sub.50/ed.sub.50, of compounds displaying neurotoxicity, etc., and achieves good anticonvulsive activity and acceptable neurotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
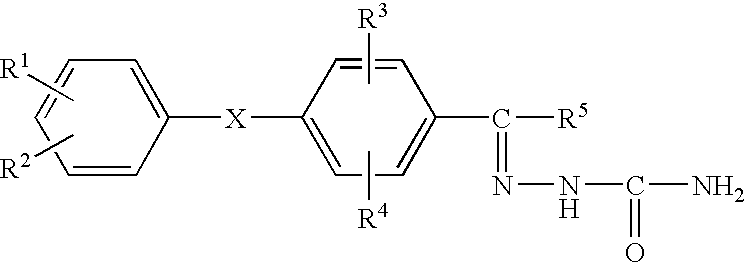
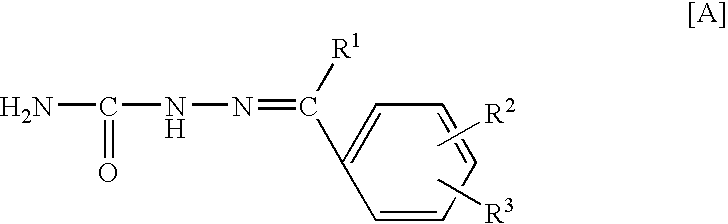
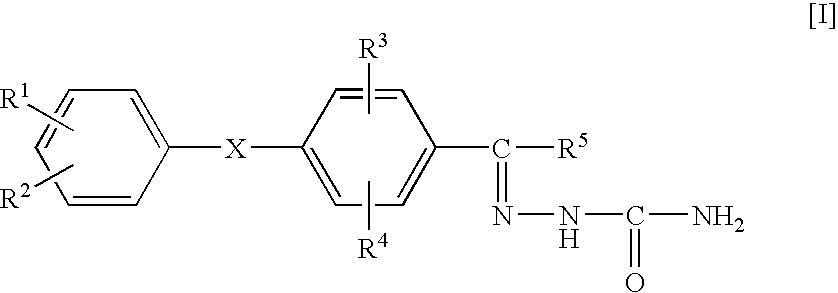
The compounds 2a to 5v shown in Table 1 below were synthesized by the method previously mentioned. The structures of the listed compounds correspond to those shown in FIG. 2 identified by the same first number (2, 3, 4 or 5), with only the substituents being identified in Table 1.
TABLE 1 Aryl Substituents, Physical Data and Anticonvulsant Evaluation after Intraperitoneal Injection into Mice and Oral Administration to Rats of the Compounds in Series 2-5 intraperitoneal injection in mice.sup.a MES acPTZ toxicity oral administration to rats.sup.b aryl yield screen screen screen dose MES screen compound substituents m.p.(.degree. C.) % 0.5 h 4 h 0.5 h 4 h 0.5 h 4 h (mg / kg) 0.25 h 0.5 h 1 h 2 h 4 h 2a H 198-199 40 -- -- -- -- -- -- 50 -- -- 2 1 1 2b 4-F 210-212 48 -- -- -- -- -- -- 30 0 0 1 2 4 3 H 224-225 70 -- 300 -- -- -- -- 50 -- -- -- -- -- 4a H 224-225 60 100 300 -- -- -- -- 50 -- 3 4 4 4 4b 4-F 233-234 65 30 100 -- -- -- -- 50 2 4 4 4 4 4c 4-Cl 225-226 40 30 30 30 -- 300 30 50 4 4...
example 2
An initial anticonvulsant evaluation of the compounds prepared according to Example 1 was undertaken by administering the compounds by the intraperitoneal route to mice. Protection and / or neurotoxicity was noted 0.5 and 4 hours after administering doses of 30, 100 and 300 mg / kg of each semicarbazone to the animals. These results are presented in Table 1 above.
All of the compounds were active in the MES screen except compounds 2a,b,5t,v and protection was afforded by 60% of the compounds in the scPTZ test. Neurotoxicity was displayed by approximately 70% of the semicarbazones. Bioactivity was quantitated for selected compounds and these data are given in Table 2 below:
TABLE 2 Evaluation of Selected Compounds in the MES, acPTZ and Neurotoxicity Screens after Intraperitoneal Injection in Mice MES screen acPTZ screen neurotoxicity screen PI Com- pound t (h) ED.sub.50 (mg / kg) (95% CI) slope (SE) t (h) ED.sub.50 (mg / kg) (95% CI) slope (SE) t (h) TD.sub.50 (mg / kg) (95% CI) slope (SE) ##EQU...
example 3
The compounds having the structures shown in Table 4 were prepared. The structures of the listed compounds correspond to those shown in FIG. 3 identified by the same first number (12, 13, 14, 15, 16, 17 or 18), with only the substituents being identified in Table 4.
TABLE 4 Aryl Substituents, Physical Data and Anticonvulsant Evaluation after Intraperitoneal Injection into Mice and Oral Administration to Rats of the Compounds in Series 12-18.sup.a intraperitoneal injection in mice.sup.b MES acPTZ toxicity oral administration to rats.sup.c m.p. yield screen screen screen dose MES screen compound R.sup.1 R.sup.2 (.degree. C.) % 0.5 h 4 h 0.5 h 4 h 0.5 h 4 h (mg / kg) 0.25 h 0.5 h 1 h 2 h 4 h 12a H F 240.sup.a 65 30 100 -- -- -- -- 50 2 4 4 4 4 12b H H 224-225 60 100 300 -- -- -- -- 50 -- 3 4 4 4 12c H Cl 225-226 40 30 30 30 -- 300 30 50 4 4 4 4 4 12d H Br 225-226 60 30 30 -- -- 300 30 50 1 4 4 4 4 12e H CH.sub.3 219-221 50 30 100 -- -- -- -- 50 3 4 4 4 4 13a CH.sub.3 H 169-171 60 30 100 -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com