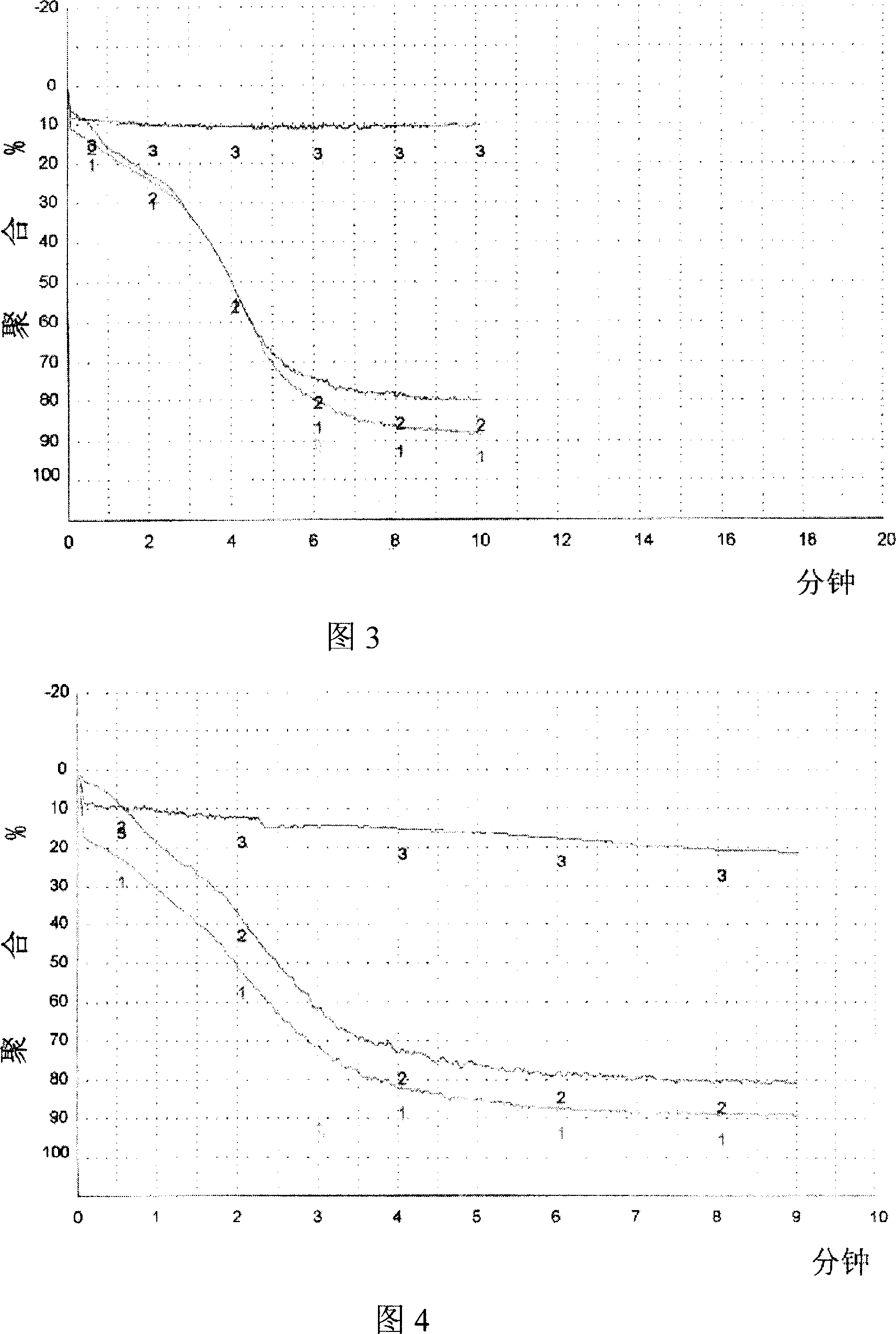
Improvement of freezing platelet storage
A technology for platelets and whole blood, applied in the direction of medical raw materials derived from mammals, etc., can solve the problems of platelets cannot be transfused into platelets, and platelets cannot be stored for a long time, and achieve the effects of avoiding bacterial reproduction, good hemostatic function, and good curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
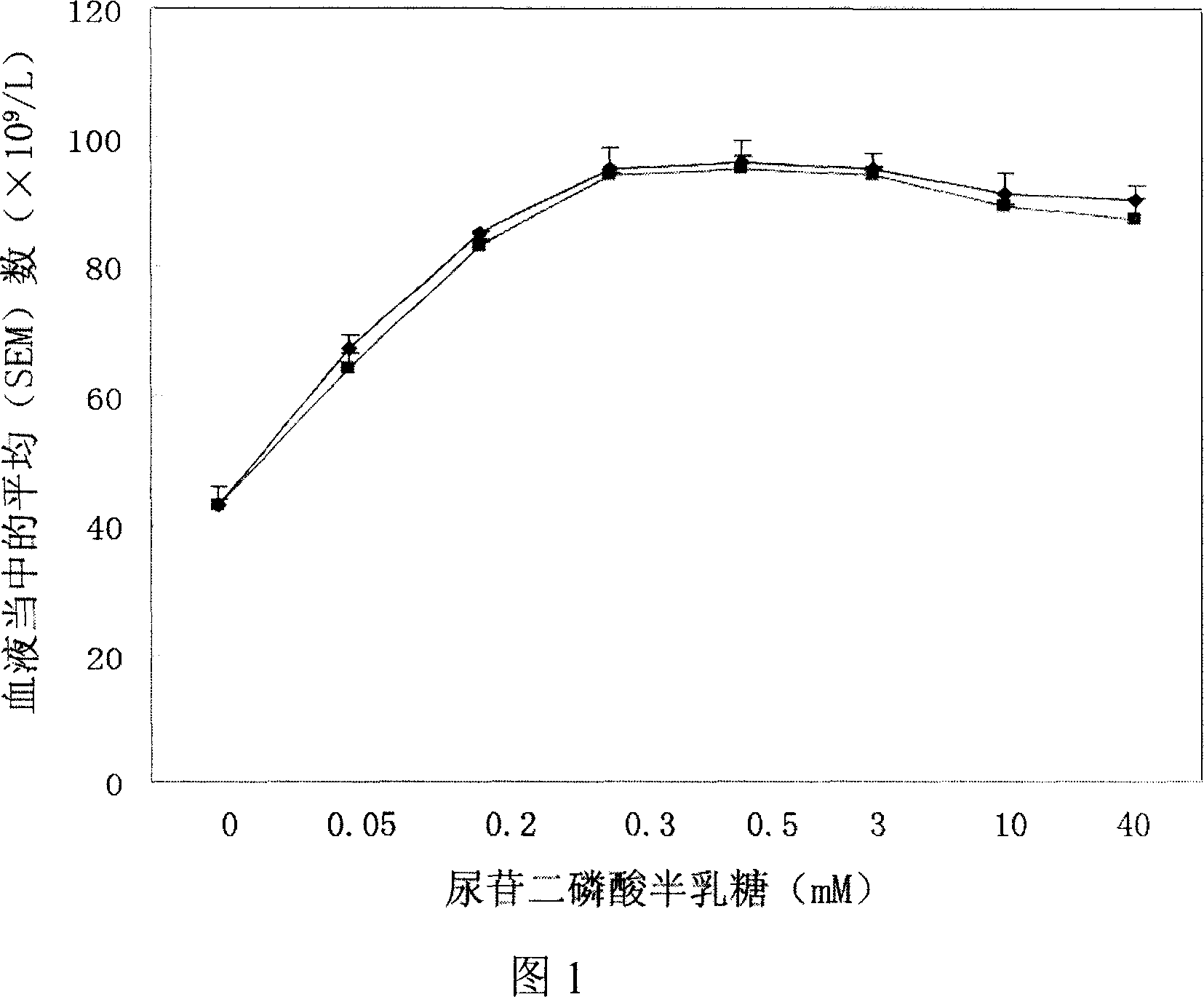
[0066] Preparation Example 1 Selection of galactose uridine diphosphate concentration
[0067] Platelet counts 1 hour after infusion are described corresponding to the concentration of added galactose uridine diphosphate. The blue line represents platelets stored at 0°C, and the red line represents platelets stored at -80°C. Test results, see Figure 1. When the concentration of galactose uridine diphosphate is 50μM, the average (SEM) number in the blood is greater than 75×10 9 / L; when the concentration of galactose uridine diphosphate was 300 μM, the average (SEM) number in the blood was 98.3×10 9 / L; when the concentration of galactose uridine diphosphate was greater than 300 μM, the average (SEM) number in the blood was 98×10 9 / L or so, there is no obvious change. Experiments show that the concentration of galactose uridine diphosphate is preferably 50 μM-50 mM, more preferably 100 μM-3 mM, and most preferably 300 μM.
preparation example 2
[0068] Preparation Example 2 Preparation of Platelet Concentrate
[0069] Processing of whole blood:
[0070] Human whole blood was obtained from healthy volunteers. The blood product was equally divided into 5 aliquots: two of them were treated with 300 [mu]M galactose uridine diphosphate for 30 minutes at 37[deg.]C.
[0071] Procurement of Platelet Concentrate: Platelet concentrates are prepared from blood collected into the ACD. Add 0.8 μM prostaglandin I 2 Then, it was collected by centrifugation at 900×g for 15 minutes.
[0072] Processing of Platelet Concentrate:
[0073] Platelet concentrate obtained from whole blood without galactose uridine diphosphate:
[0074] Sample C1 was stored at room temperature (fresh platelets);
[0075] Sample C2, stored at 0°C for 2 hours
[0076] For sample C3, 5% (final concentration) of DMSO was added, and then stored at -80°C for 2 hours.
[0077] Platelet concentrate obtained by adding galactose uridine diphosphate whole blood:...
preparation example 3
[0081] Preparation Example 3 Preparation of Platelet Suspension
[0082] Acquisition of platelet suspension: the platelet concentrate obtained as described above was centrifuged at 1500×g for 10 minutes to remove the supernatant to obtain platelet pellet, and then washed with CGS buffer (13mM sodium citrate, 30mM D-glucose, 120mM sodium chloride ) wash 2 times. The eluted platelets were resuspended in Tyrode's buffer (8.19 g sodium chloride, 0.20 g potassium chloride, 1.01 g sodium bicarbonate, 0.055 g sodium dihydrogen phosphate, 0.991 g D-glucose) to obtain a platelet suspension.
[0083] Processing of platelet suspension:
[0084] Platelet suspension obtained without adding galactose uridine diphosphate whole blood:
[0085]Sample S1 was stored at room temperature;
[0086] Sample S2 was stored at 0°C for 2 hours;
[0087] Sample S3 was added with 5% (final concentration) DMSO, and then stored at -80°C for 2 hours.
[0088] Platelet suspension obtained by adding galact...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap