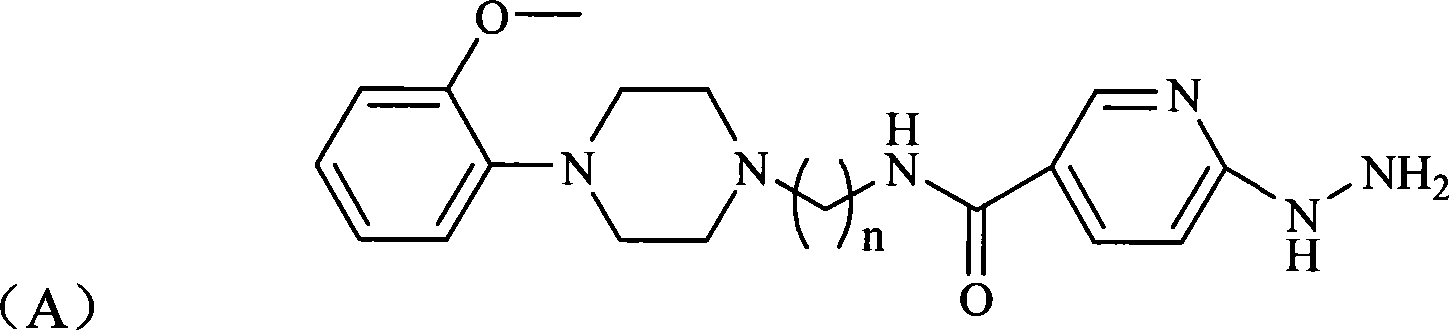99mtc marked 2-methoxyphenyl piperazine derivative complexes as well as preparation method and uses thereof
A technology of methoxyphenyl and 99mtc, which is applied in the field of radioactive complexes, can solve the problems of low brain uptake, strong fat-solubility, and poor specificity of complexes, and achieve simple preparation methods, excellent biological properties, and low cost of preparation and use low effect
Inactive Publication Date: 2010-11-24
BEIJING NORMAL UNIVERSITY
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
More importantly, although these 99mTc-labeled complexes show high receptor affinity in vitro, the in vivo experimental results are not satisfactory, mainly due to low brain uptake and poor specificity. The possible reason is the lipid solubility of the complexes. Too strong and too much molecular weight, etc.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
The invention relates to a 2-methoxy benzene group piperazine derivative complex marked by radioactive <99m>Tc. The 2-methoxy benzene group piperazine derivative complex considers the <99m>Tc as a central core; one ligand is 2-methoxy benzene group piperazine derivative compound, the structure of which is shown in a formula (A), wherein, n is an integer of 1 to 6; carbon chain length is shown as C1 to C6; the other ligand is one kind of nitrilotriacetic acid, tris (hydroxymethyl) methyl glycine, di (hydroxymethyl) methyl glycine or hydroxyethylethylenediaminetriacetic acid. The complex can beused as 5-HT1A brain receptor imaging agent and has the advantages of simple preparation, low price, high target / non-target ratio etc. The invention also relates to a preparation method of the complex and an application of the complex as the 5-HT1A brain receptor imaging agent.
Description
A 99mTc-labeled 2-methoxyphenylpiperazine complex and its preparation method and application technical field The invention relates to a 99mTc-labeled radioactive complex, especially a 99mTc-labeled 2-methoxyphenylpiperazine complex, a preparation method thereof and application as a 5-HT1A brain receptor imaging agent. Background technique Functional imaging of central nervous system (CNS) receptors has become an important field in radiopharmaceutical research. Changes in neuroreceptor density and function are closely related to neurological and psychiatric disorders (GrossC, ZhuangX, StarkK, et al. SerotoninlAreceptoracts during development to establish normal laxiety-like behavior in an adult [J]. Nature, 2002, 416(6879): 396). In particular, the subtype 5-HT1A of the central neurotransmitter 5-hydroxytryptamine (5-HT) receptor is related to insomnia, anxiety, depression, schizophrenia and senile dementia (Alzheimer's disease). 5-HT1A receptor is a kind of G-protein coup...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07F13/00A61K51/04A61K103/10
Inventor 张现忠范卫卫林艳庞燕唐志刚张俊波王学斌陆洁
Owner BEIJING NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com