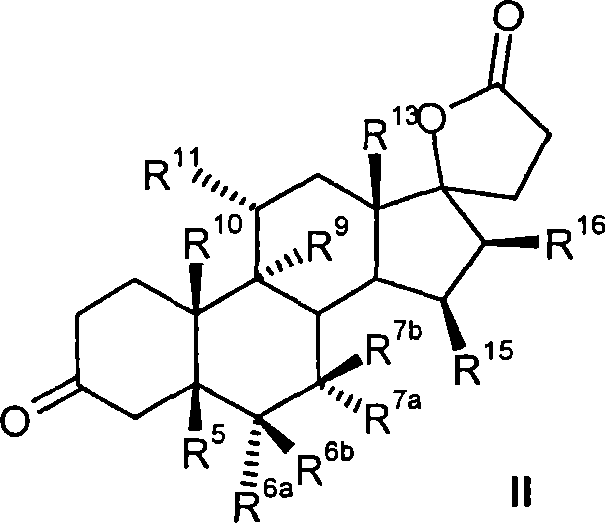
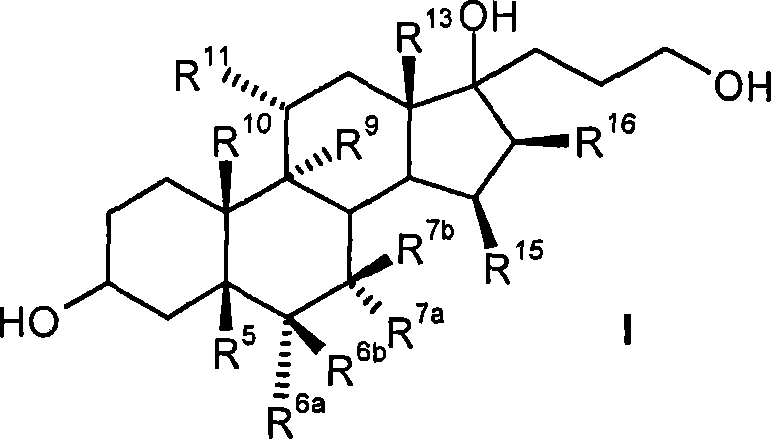
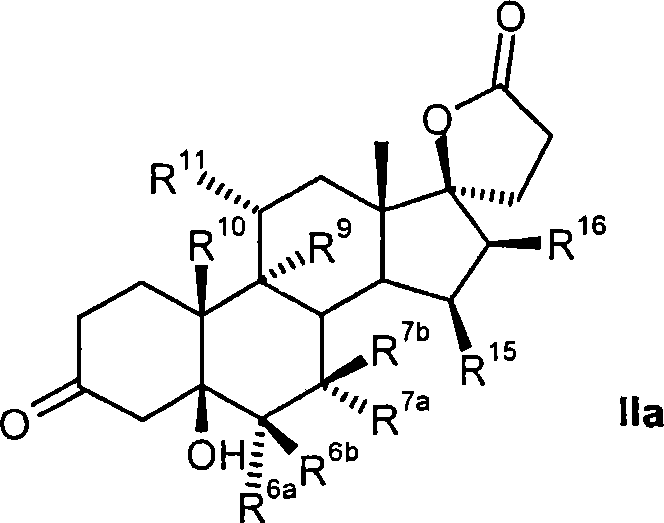
Process for the production of 3-oxo-pregn-4-ene-21,17-carbolactones by the metal-free oxidation of 17-(3-hydroxypropyl)-3,17-dihydroxyandrostanes
A technology of oxo and lactone, applied in the field of 6β, can solve the problems of costly, laborious and expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1 6β,7β; 15β,16β-dimethylene-3-oxo-17α-pregnane-5β-ol-21,17-lactone-dichloromethane hemisolvate (IV):
[0097] According to GOP1, 30 g (0.0769 mol) of 17α-(3-hydroxypropyl)-6β,7β;15β,16β-dimethylene-androstane-3β,5β,17β-triol were reacted.
[0098] During the reaction, the product 6β,7β;15β,16β-dimethylene-3-oxo-17α-pregnane-5β-ol-21,17-lactone accumulated in the form of its dichloromethane hemisolvate . After destruction of excess oxidant and after work-up according to GOP1, 27 g of 6β,7β;15β,16β-dimethylene-3-oxo-17α-pregnane-5β-ol-21,17 were isolated -Lactone-methylene chloride hemisolvate (0.0630 mol) = 82% of theory.
[0099] [α] D 20 =-61° (c=1.0; CHCl 3 ); Melting point = 121°C;
[0100] 1 H-NMR (400MHz, CDCl 3 ): δ = 0.52 (q J = 5.5Hz, 1H, 21α-H [15, 16-methylene bridge]), 0.68-0.78 (m, 2H, 20-H [6, 7-methylene bridge]), 0.89-0.97(m, 1H, 6-H), 0.93(s, 3H, 19-H), 0.99(s, 3H, 18-H), 1.19-1.52(m, 7H), 1.54-1.85(m, 6H), 1.92 (dd J=3.8 and 11.8Hz, 1...
Embodiment 2
[0104] Example 2 6β, 7β; 15β, 16β-dimethylene-3-oxo-17α-pregna-4-ene-21,17-lactone (IIIb):
[0105] According to GOP2, 30 g (0.0769 mol) of 17α-(3-hydroxypropyl)-6β,7β;15β,16β-dimethylene-androstane-3β,5β,17β-triol were reacted. After destroying excess oxidizing agent according to GOP2, the reaction mixture was acidified to pH 1 with 10% sulfuric acid solution and stirred at 25° C. for 30 minutes. After working up according to GOP2, 21.5 g of 6β,7β;15β,16β-dimethylene-3-oxo-17α-pregna-4-ene-21,17-lactone (0.059 mol)= 76.7 Theoretical%.
[0106] [α] D 22 ≈-182°(c=0.5 CHCl 3 ); melting point = 201.3°C; UV(MeOH): ε 265 = 19,000; more important 1 H-NMR data (CDCl 3 ): δ=0.40-0.67(m, 1H, cyclopropyl H), 1.01(s, 3H, 18-H), 1.11(s, 3H, 19-H), 6.04(s, 1H, 4-H) (D. Bittler, H. Hofmeister, H. Laurent, K. Nickisch, R. Nickolson, K. Petzoldt, R. Wiechert; Angew. Chem. Int. Ed. Engl. 1982, 21, 696-697];
[0107] MS (EI, 70eV) m / e=366 (M + ); m / e=338(M + -CO); m / e=351 (M + -CH ...
Embodiment 3
[0108] Example 3 6β, 7β: 15β, 16β-dimethylene-3-oxo-17α-pregna-4-ene-21,17-lactone (IIIb):
[0109]According to GOP3, make 30 g (70, 25 mmol) of 6β,7β:15β,16β-dimethylene-3-oxo-17α-pregnane-5β-ol-21,17-lactone dichloromethane hemisolvent Compound (from Example 1) was reacted to obtain 24.30 g of drospirenone (yield: 94.5%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com