A method for making anode material LiFePO4 of lithium ion battery
A lithium ferrous phosphate, lithium-ion battery technology, applied in electrode manufacturing, battery electrodes, chemical instruments and methods, etc., can solve the limitations of large-scale industrialization of lithium ferrous phosphate, poor high-current discharge capability of synthetic materials, and control conditions. Harsh and other problems, to achieve the effect of reducing synthesis cost, good high current, and short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
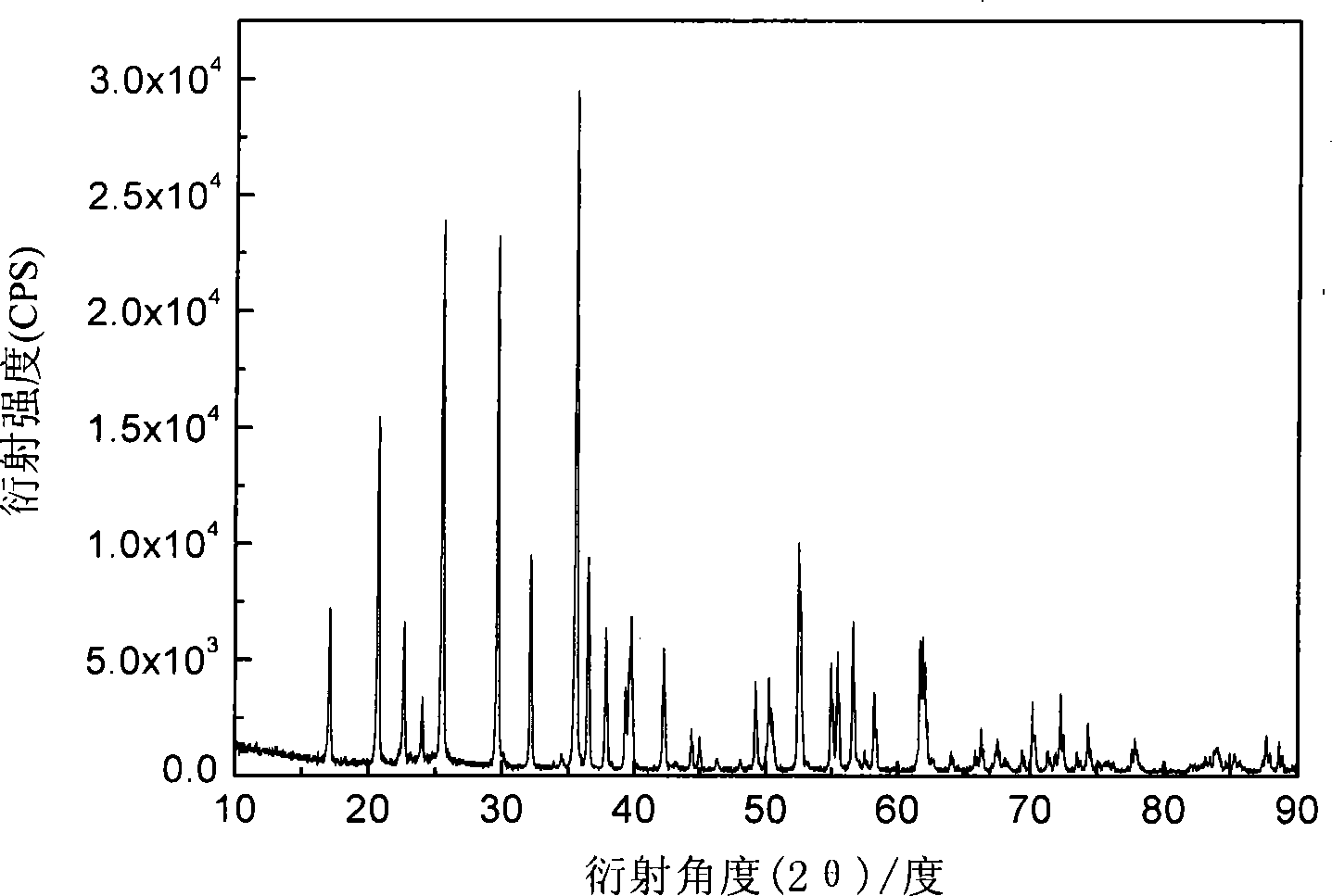
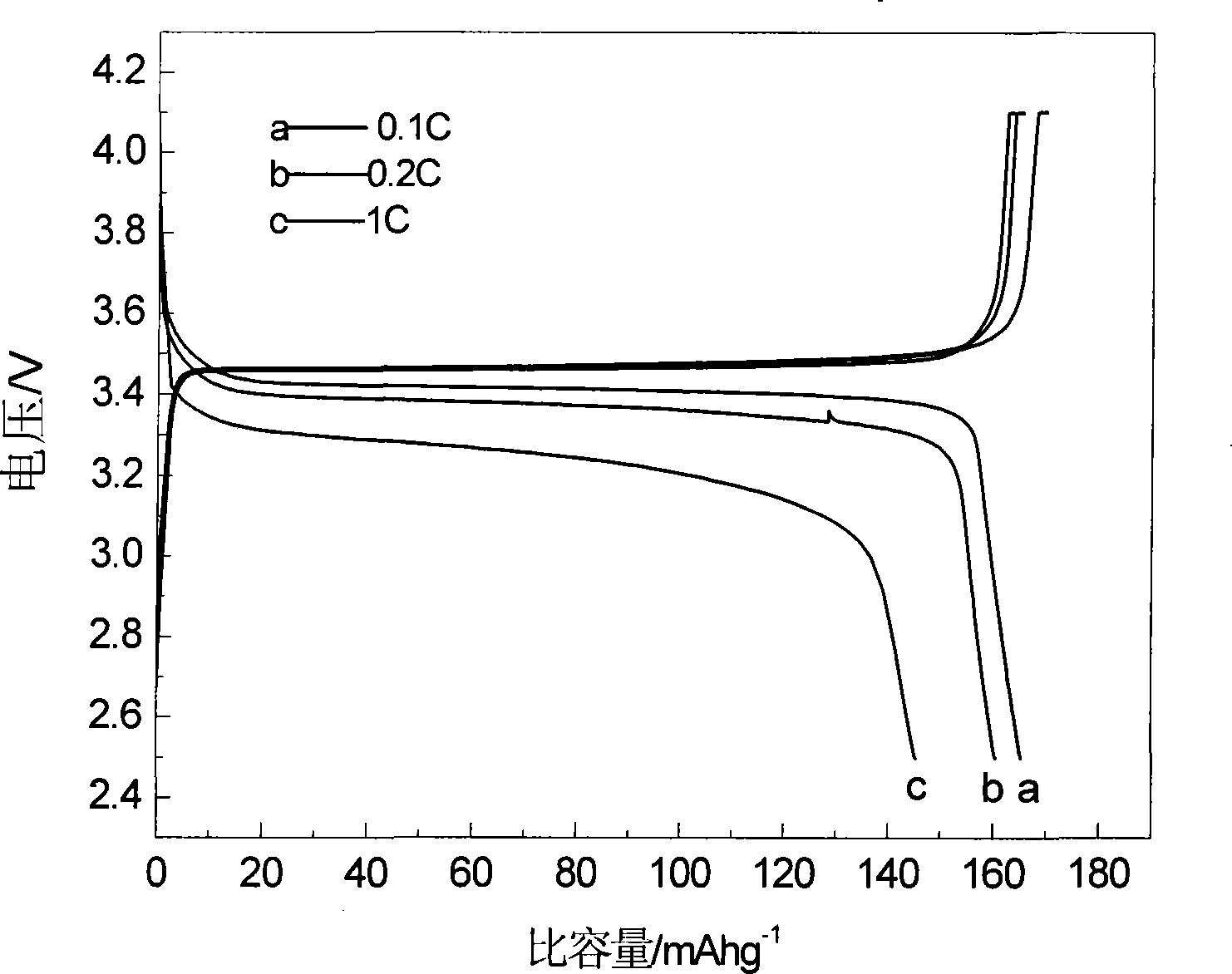
[0018] Using iron phosphate, lithium carbonate and malic acid as raw materials, the molar ratio of 1:0.5:1 is mixed uniformly, and mechanically activated for 0.5 hours; then it is put into a tube furnace, and the temperature is 300℃ and 500℃ respectively under argon atmosphere. Constant temperature at ℃, 560℃, 700℃ for 12 hours. The obtained material has an olivine structure by X-ray diffraction analysis, and the space group is Pnma, which is LiFePO 4 Structure. The particle size of the product can be obtained by SEM at about 200nm. The resulting products were assembled into button batteries to test their charge-discharge specific capacity and cycle performance. They were charged and discharged at a rate of 0.1C. Their first discharge capacity and discharge capacity after 50 cycles are shown in Table 1.
[0019] Table 1 Experimental conditions and results of Example 1
[0020]
Embodiment 2
[0022] Using ferric nitrate, lithium formate, triamine phosphate, and mandelic acid as raw materials, mix homogeneously at a molar ratio of 1:1:1:3, and mechanically activate for 20 hours; then put it into a tube furnace, under hydrogen atmosphere, temperature Keep at 600°C for 2 hours, 5 hours, 8 hours, and 20 hours. The obtained material has an olivine structure by X-ray diffraction analysis, and the space group is Pnma, which is LiFePO 4 Structure. The particle size of the product can be obtained by SEM at about 200nm. The resulting products were assembled into button batteries to test their charge-discharge specific capacity and cycle performance. They were charged and discharged at a rate of 0.1C. Their first discharge capacity and discharge capacity after 50 cycles are shown in Table 2.
[0023] Table 2 Experimental conditions and results of Example 2
[0024]
Embodiment 3
[0026] Using iron carbonate, lithium oxide, diammonium hydrogen phosphate, and oxalic acid as raw materials, mix homogeneously with a molar ratio of 1:1:2:4, and mechanically activate for 8 hours; then put it into a tube furnace, under a nitrogen atmosphere, The temperature is kept at 560°C for 15 hours. The obtained material has an olivine structure by X-ray diffraction analysis, and the space group is Pnma, which is LiFePO 4 Structure. Assemble the obtained product into a button cell to test its charge-discharge specific capacity and cycle performance, charge and discharge at a rate of 0.1C, the first discharge capacity is 165mAh·g -1 , Discharge capacity 165mAh·g after 50 cycles -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com