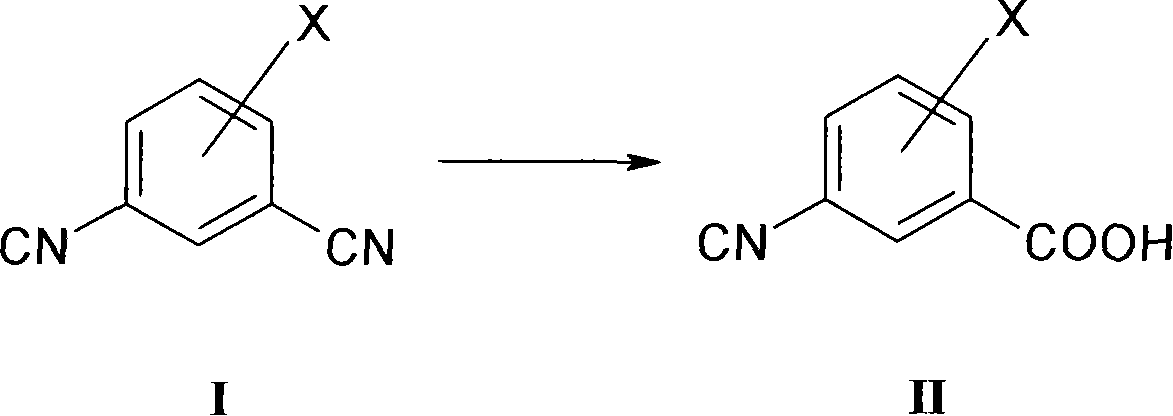
New process for converting aromatic halo-substituted dinitriles into halo-substituted cyanocarboxylic acids
A technology of carboxylic acid and amino acid, applied in fermentation, hydrolase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0046] 1. Preparation of Cell-Free Extract (CFE) Biocatalyst
[0047] 1 liter of Terrific Broth (TB) medium supplemented with carbenicillin (100 mg / l) was inoculated with a glycerol stock of E. coli DH 10B (pMS470-3-14-1-4 from Rhodococcus rhodochrous nitrilase). After 68 hours of growth, this culture was used to inoculate 9 liters of TB medium supplemented with carbenicillin (100 mg / l) and IPTG was used as inducer. After 41 hours of growth in laboratory-grade fermenters, fully grown fermentation broth (OD 600 = 16.7) Harvest the cells, resuspend the cell wet weight biomass into 20 mM HEPES / NaOH buffer (pH 7.0, 13 μg benzonase enzyme per gram wet weight cells) at the ratio of 1 kg cells + 3 kg buffer, and transfer Cells were disrupted at 1600 bar in a (nanojet) (two runs). 1.04 liters of CFE were obtained with a yield of 86% (328 g wet biomass + 880 ml buffer).
[0048] 2. Enzyme conversion
[0049] 1.5 mol of 5-fluoro-1,3-dicyanobenzene was suspended in 81 0.1M sodium pho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com