Alkoxy indolinone based protein kinase inhibitors
An alkoxy, alkyl technology, applied in the field of protein kinase inhibitors, can solve problems such as doubts, poor water-soluble drug properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1-8
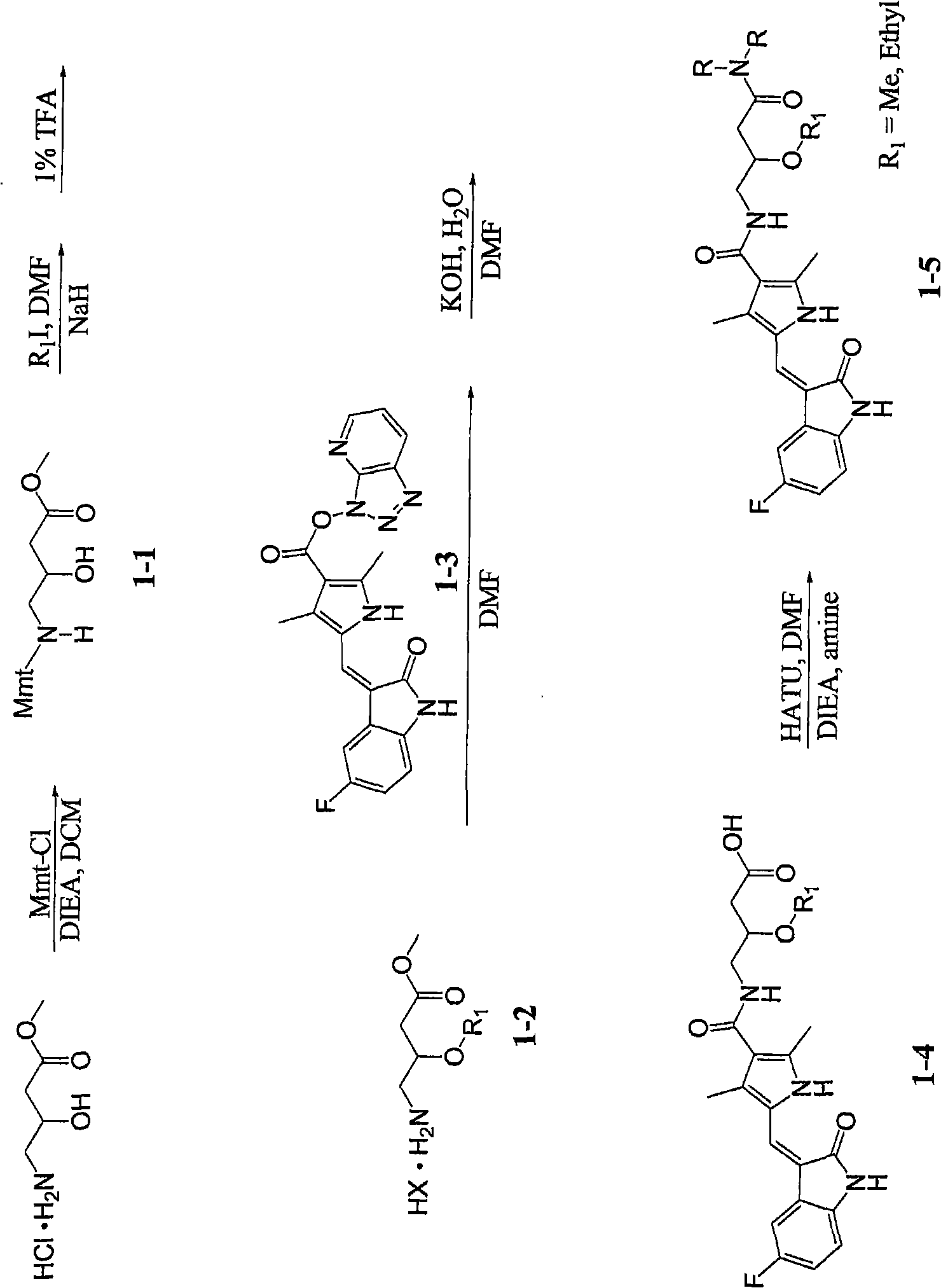
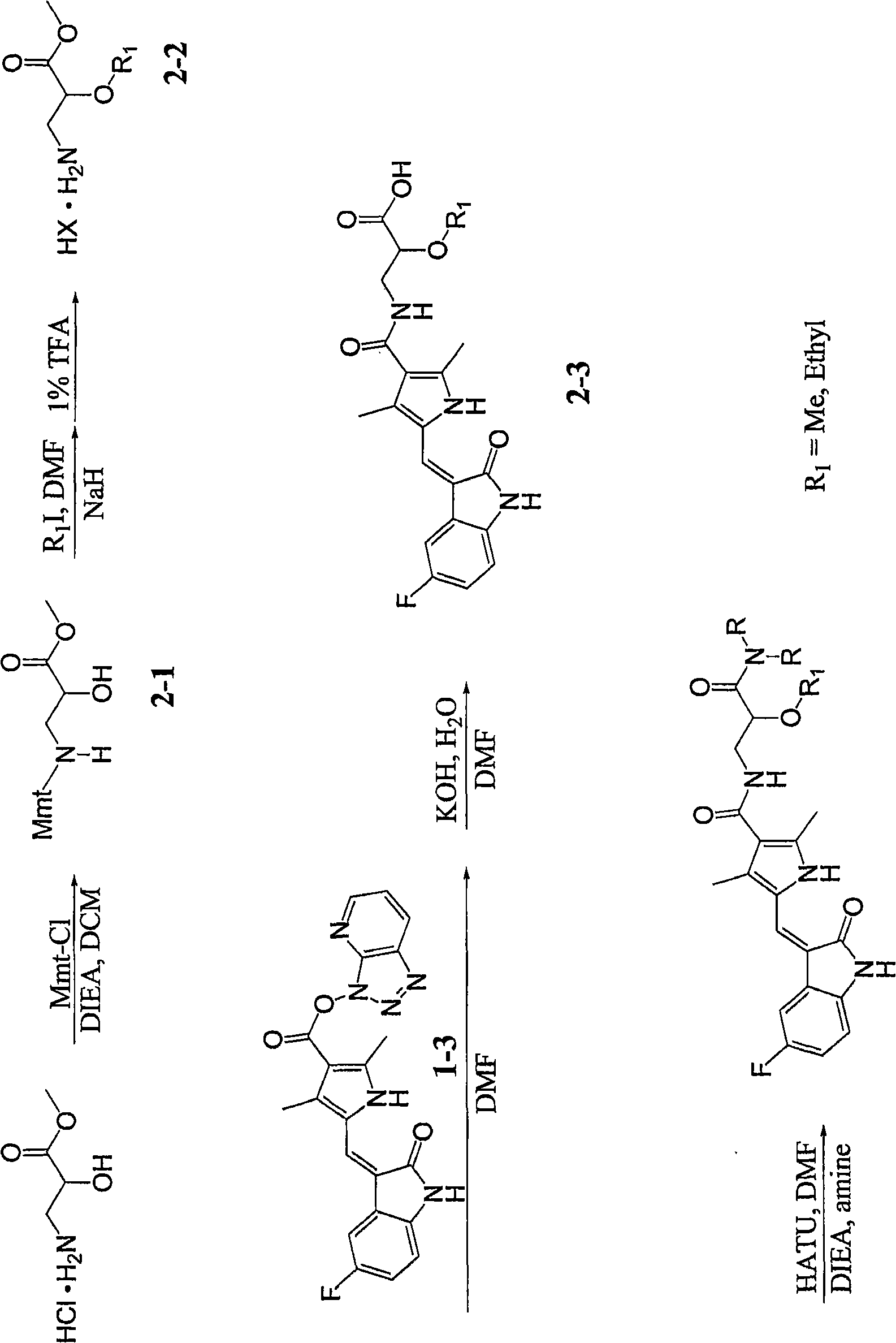
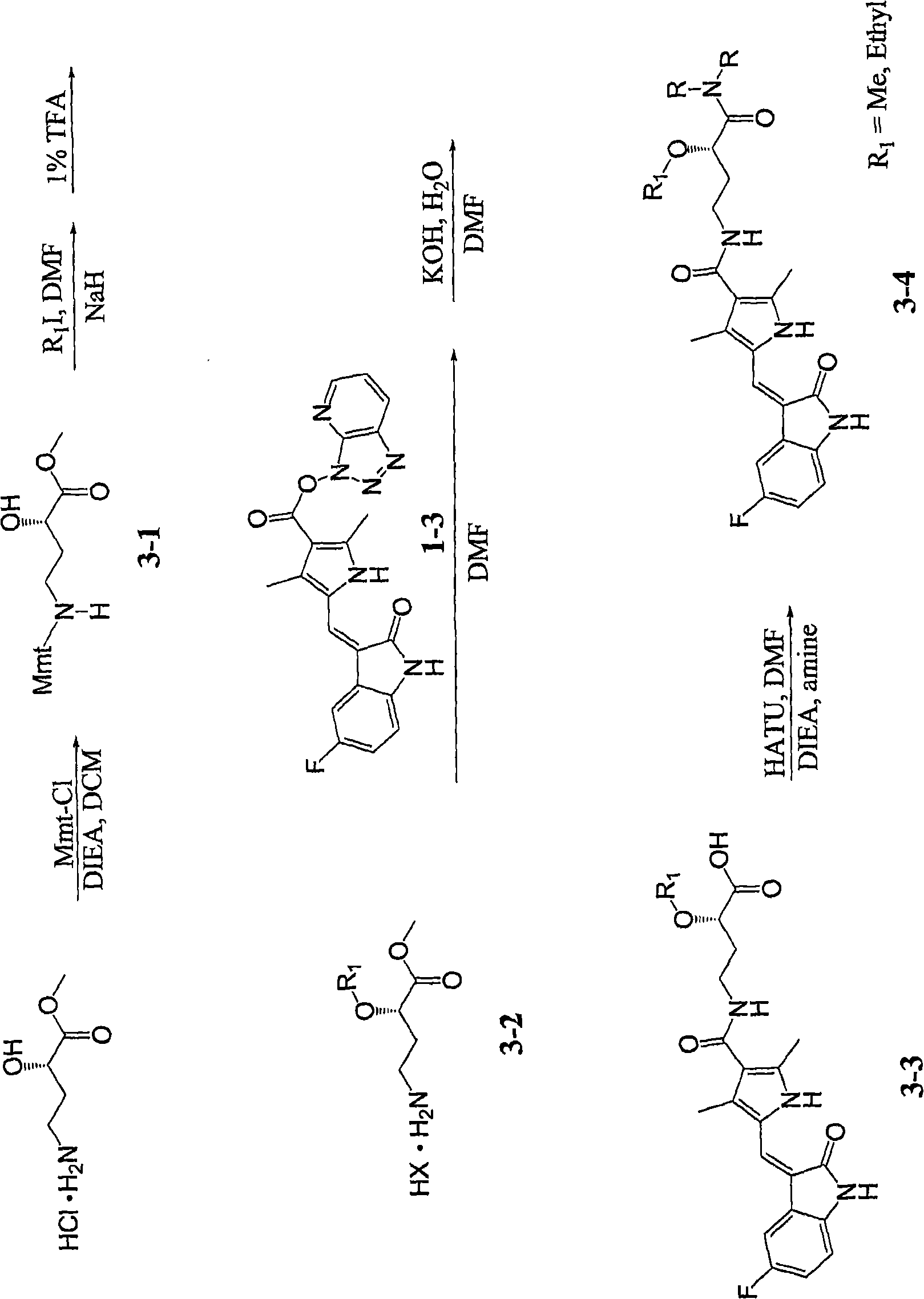
[0030] Example 1-8: the synthesis of acid (1-4) and amide (1-5) as figure 1 shown. Variations of this general synthetic procedure can be understood and performed by those skilled in the art, and thus the compounds of the present invention can be synthesized by such skilled artisans.
example 1
[0031] Example 1: 4-({5-[5-fluoro-2-keto-1,2-dihydro-indole-(3Z)-methylenemethyl]-2,4-dimethyl-1H-pyrrole -3-Carbonyl}-amino)-3-methoxy-butanoic acid
[0032]
[0033] To a solution of methyl 4-amino-3-hydroxybutyric acid (1.0 equiv, prepared by refluxing the free amino acid in anhydrous methanol containing 1.2 equiv of HCl) and DIEA (5 equiv) in DCM was added Mmt- CI (1.1 equiv). After stirring overnight, DCM was removed by distillation under reduced pressure. The residue was suspended in ethyl acetate, washed with brine (3x) and washed with anhydrous Na 2 SO 4 dry. Thereafter, ethyl acetate was removed, and the residue was dried overnight in high vacuum, and compound 1-1 was obtained by flash chromatography. To a solution of compound 1-1 in anhydrous DMF was added NaH (1.5 eq.) under argon atmosphere. After stirring at 25°C for 1 hour, MeI (5 equiv) was added to the solution, and the resulting suspension was shaken gently at 25°C overnight. DMF was removed under va...
example 2
[0034] Example 2: 3-ethoxy-4-({5-[5-fluoro-2-keto-1,2-dihydro-indole-(3Z)-ylidenemethyl]-2,4-di Methyl-1H-pyrrole-3-carbonyl}-amino)-butyric acid
[0035]
[0036] The title compound was prepared using a very similar procedure to the compound in Synthesis Example 1, except that ethyl iodide was used instead of methyl iodide to obtain the 3-ethoxy compound (9.7% based on compound 1-3). LC-MS: 254nm singlet, MH + use C 22 h 24 FN 3 o 5 Calculated value: 430, actual obtained value: 430.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com