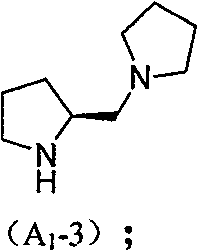
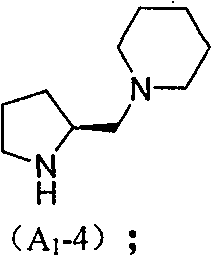
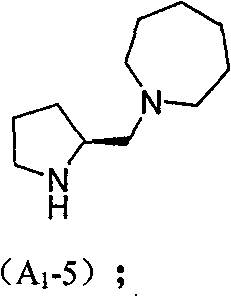
Chiral organic micromolecule catalyst loaded by heteropoly acid and preparation method and use thereof
A technology for small molecules and heteropolyacids, which is applied in the field of chiral small molecule catalysts and their preparation, can solve the problems of reduced activity and selectivity, reduced activity, and cumbersome catalyst preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: synthetic A 1 -1
[0061]
[0062] N 2 Under cooling in a protective ice bath, slowly add 10.6 g of DCC (56.4 mmol) in 20 mL of dichloromethane solution in 30 mL of dichloromethane solution dissolved with 12.7 g (51.3 mmol) of N-Z protected proline, and stir for 30 minutes. Then slowly add dichloromethane solution containing 3.75 g (51.3 mmol) diethylamine dropwise, react at room temperature for 12 hours, add a small amount of water to consume excess DCC, filter to remove the white solid generated, and the filtrate is successively washed with 2% hydrochloric acid 20mL, 4% Wash with 20 mL of sodium bicarbonate, 20 mL of saturated saline, and 20 mL of distilled water, dry over anhydrous sodium sulfate, spin off the solvent, separate by column chromatography to obtain 7.5 g of a colorless viscous liquid, add it to a dry two-necked bottle, and add 0.4 g of Pd / C catalyst, pump vacuum, inject 30mL of anhydrous methanol, feed 10 atmospheres of hydrogen, rea...
Embodiment 2
[0064] Embodiment 2: synthetic A 1 -4
[0065]
[0066] Proline is amino-protected with Z-Cl, N-Z-protected proline B is condensed with hexahydropyridine under DCC to generate N-Z-protected prolinamide C, and H is catalyzed by Pd / C 2 Reduction deprotection of Z, the obtained compound D with LiA1H 4 Reduction to obtain small organic molecule A 1 -4.
[0067] Concrete synthetic steps are as follows:
[0068] N 2 Under cooling in a protective ice bath, slowly add 10.6 g of DCC (56.4 mmol) in 20 mL of dichloromethane solution in 30 mL of dichloromethane solution dissolved with 12.7 g (51.3 mmol) of N-Z protected proline B, and stir for 30 minutes , then slowly add dropwise a dichloromethane solution containing 4.36 grams (51.3 mmol) of hexahydropyridine, react at room temperature for 12 hours, add a small amount of water to consume excess DCC, filter and remove the white solid generated, and the filtrate is successively washed with 2% hydrochloric acid 20mL, 4 Wash with 2...
Embodiment 3
[0074] Embodiment 3: Synthesis A 1 -9
[0075]
[0076] E F
[0077] Using hydroxyproline as a raw material, through N-Boc protection and O-benzylation, E is obtained; E is condensed with diethylamine under the action of DCC in dichloromethane solvent to obtain compound F, and F is sequentially desorbed under acidic conditions. In addition to Boc protecting group and LiAlH 4 Reduction, to obtain small organic molecule A 1 -9.
[0078] Concrete synthetic steps are as follows:
[0079] Add 9.42 grams of E (30 mmol) to a 100 mL dry single-necked bottle, add 15 mL of dichloromethane to dissolve it, slowly add 15 mL of dichloromethane solution containing 6.49 grams (31.5 mmol) of DCC dropwise under ice bath, and react for 30 minutes after the addition is complete , slowly dropwise add 20mL dichloromethane solution containing 2.19 grams (30mmol) of diethylamine, it takes about 1 hour, after the addition is completed, it rises to room temperature, reacts...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap