Acid addition salt of dihydropyridine derivative
A kind of technology of dihydrobromide and hydrobromide, applied in the field of pharmaceutical compositions, can solve problems such as not knowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
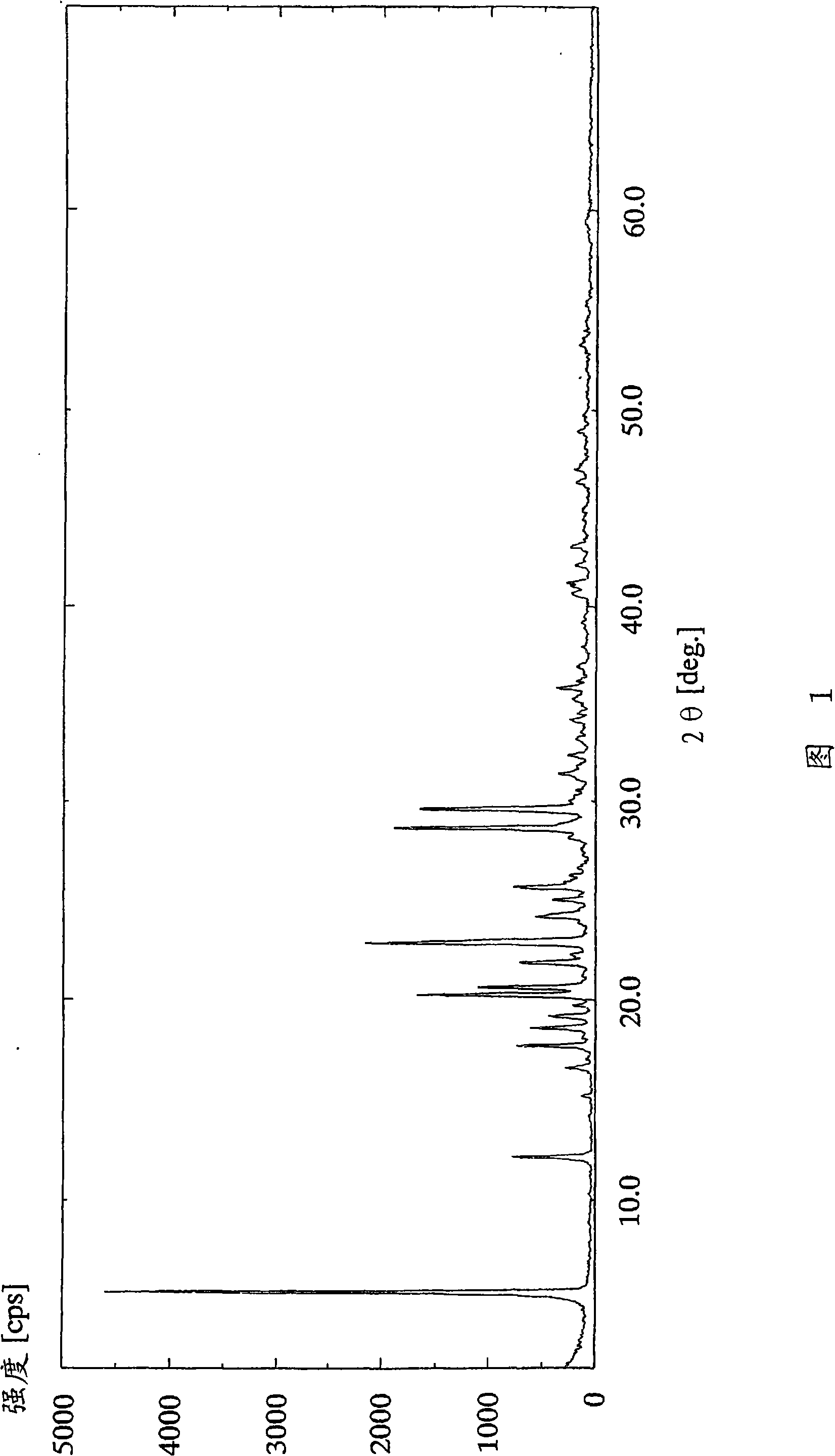
[0117] (Example 1) 2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-diphenylmethyl Azetidin-3-yl) ester 5-isopropyl dihydrobromide
[0118] In 2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-diphenylmethylazetidine To a solution of 2.91 g (5.00 mmol) of 5-isopropyl (alk-3-yl) ester in 12 ml of methanol, 1.27 ml (11.0 mmol) of 47 wt % hydrobromic acid was added dropwise at 25° C. over 30 minutes. After the dropwise addition was completed, the reaction solution was further stirred for 30 minutes. The resulting crude crystals were collected by filtration, washed with 12 ml of methanol, and dried under reduced pressure at 50° C. for 2 hours to obtain 2.90 g (78%) of the title compound as a white powder.
[0119] 1 H-NMR spectrum (DMSO-d 6 , δppm): 1.00(d; J=6Hz, 3H), 1.19(d; J=6Hz, 3H), 2.29(s, 3H), 4.01-4.38(m, 4H), 4.74-4.92(m, 2H) , 4.96-5.23(m, 1H), 5.78-6.11(m, 1H), 6.85(brs, 2H), 7.37-7.77(m, 12H), 7.94-...
Embodiment 2
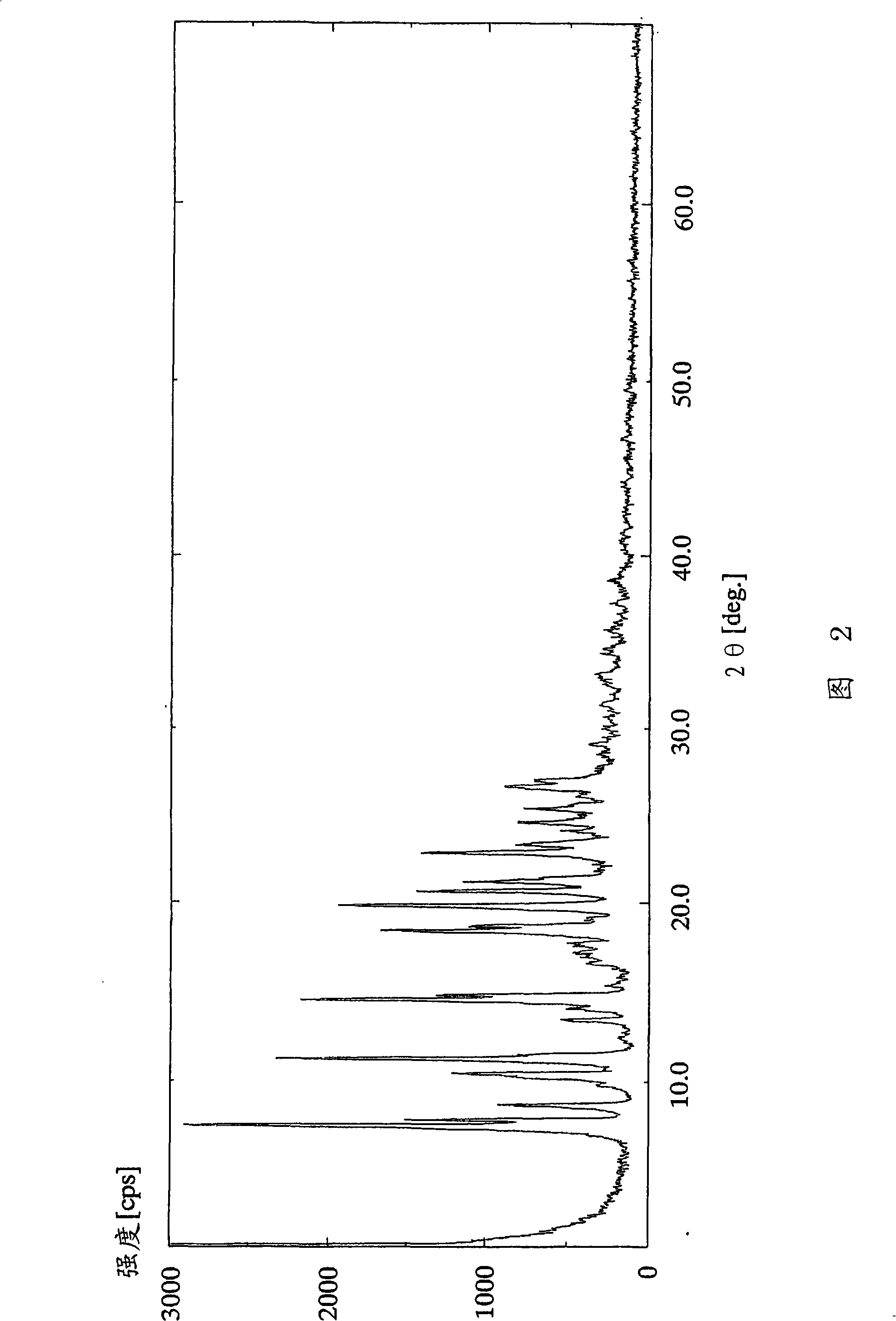
[0122] (Example 2) 2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-diphenylmethyl Azetidin-3-yl) ester 5-isopropyl ester monocitrate
[0123] In 2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-diphenylmethylazetidine To a solution of 3.24 g (5.56 mmol) of 5-isopropyl ester in 11.1 ml of acetone, 11.1 g (5.83 mmol) of a solution in 11.1 ml of acetone was added dropwise at 25° C. over 30 minutes. After the dropwise addition was completed, the reaction solution was further stirred for 40 minutes. The resulting crude crystals were collected by filtration, washed with 5 ml of acetone, and dried under reduced pressure at 50° C. for 2 hours to obtain 3.42 g (79%) of the title compound as a yellow powder.
[0124] 1 H-NMR spectrum (DMSO-d 6 , δppm): 0.99(d; J=6Hz, 3H), 1.18(d; J=6Hz, 3H), 2.27(s, 3H), 2.30-2.42(m, 1H), 2.64(d; J=15Hz, 2H), 2.75(d; J=15Hz, 2H), 2.86-2.97(m, 1H), 3.28-3.40(m, 1H), 3.49-3.58(m, 1H),...
Embodiment 3
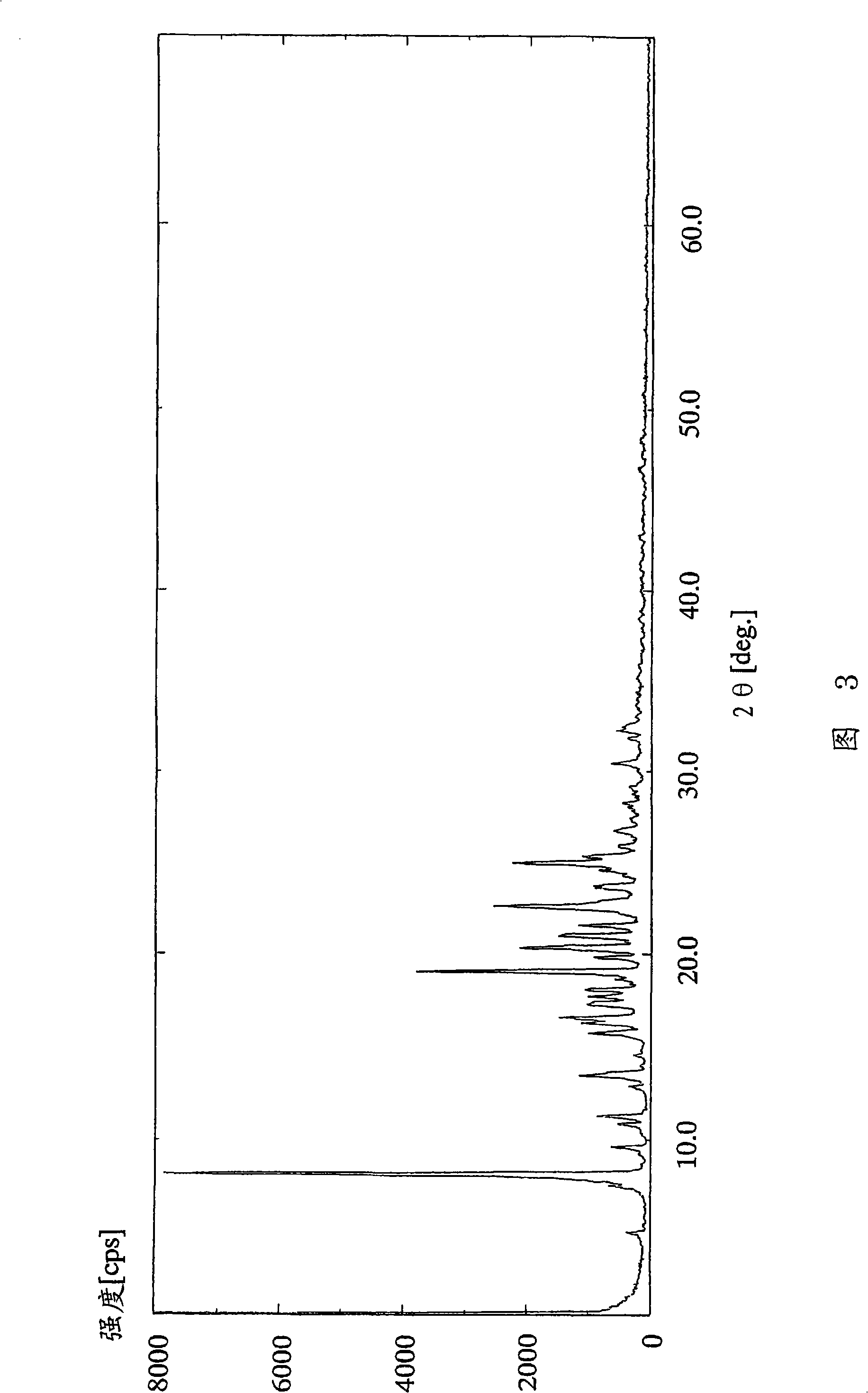
[0126] (Example 3) 2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-diphenylmethyl Azetidin-3-yl) ester 5-isopropyl dimesylate
[0127] In 2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-diphenylmethylazetidine To a solution of 4.66 g (8.00 mmol) of 5-isopropyl (alk-3-yl) ester in 32 ml of ethyl acetate, 1.04 ml (16.0 mmol) of methanesulfonic acid was added dropwise at 25°C over 30 minutes. After the dropwise addition was completed, the reaction solution was further stirred for 30 minutes. The resulting crude crystals were collected by filtration, washed with 20 ml of ethyl acetate, and dried at 40° C. under reduced pressure for 2 hours to obtain 6.01 g (97%) of the target compound as a white powder.
[0128] 1 H-NMR spectrum (DMSO-d 6 , δppm): 1.00(d; J=6Hz, 3H), 1.18(d; J=6Hz, 3H), 2.28(s, 3H), 2.38(s, 6H), 4.03-4.42(m, 4H), 4.73 -4.89(m, 2H), 4.96-5.18(m, 1H), 5.73-6.01(m, 1H), 6.85(brs, 2H), 7.37-7.67(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com