Acid addition salt of optically active dihydropyridine derivative
A dihydrobromide, dihydrochloride technology, applied in the treatment or prevention of hypertension or heart disease, or kidney damage, treatment or prevention of hypertension. , Most preferred for hypertension, more preferred in the field of hypertension or heart disease, can solve problems such as unknown acid addition salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
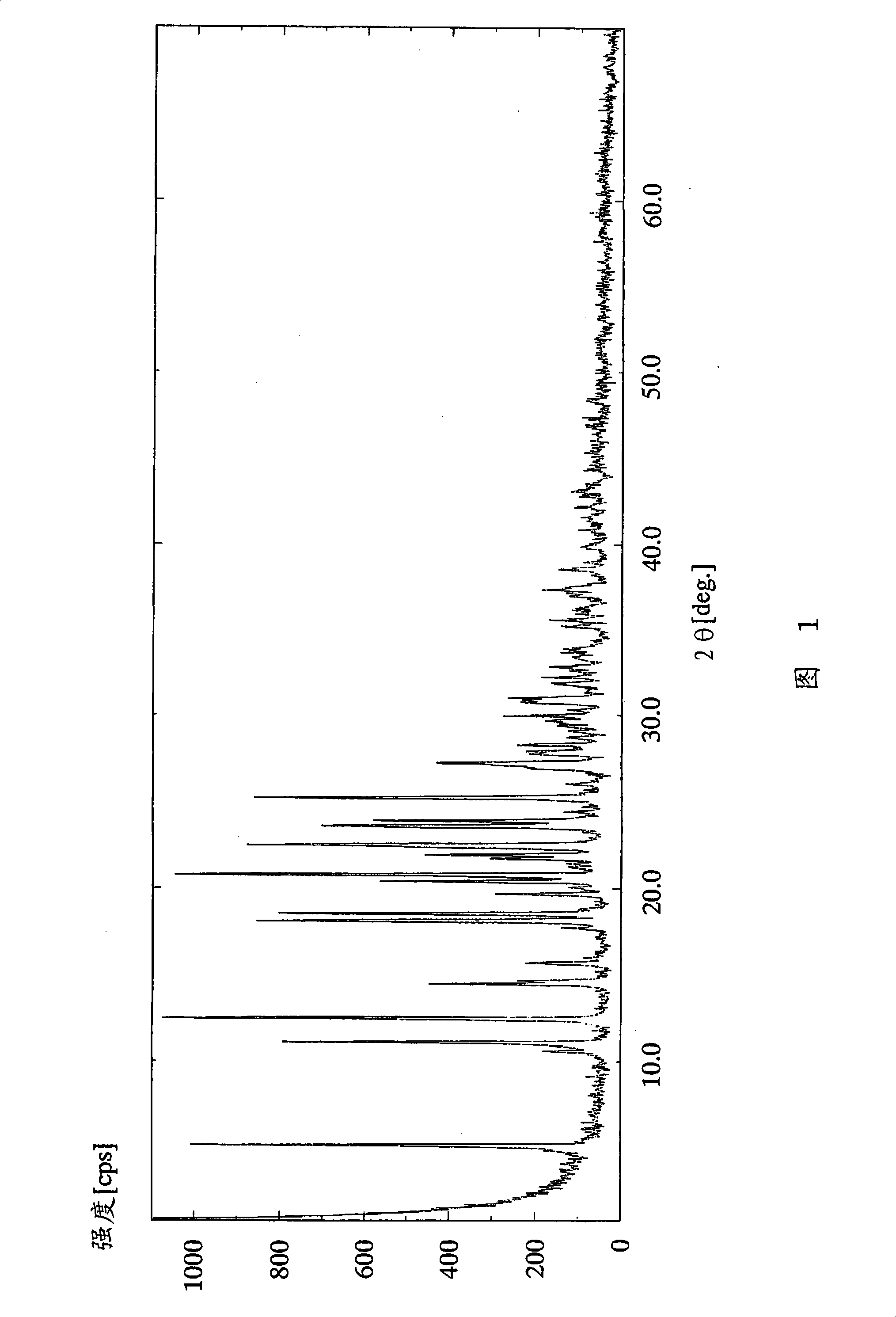
[0107] (Example 1) (R)-2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-di Phenylmethylazetidine-3-yl) ester 5-isopropyl ester dihydrochloride dihydrate
[0108]In (R)-2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-diphenylmethyl To a 240 ml ethyl acetate solution of 8.74 g (15.0 mmol) of azetidin-3-yl) ester 5-isopropyl ester, 2.57 ml (30.0 mmol) of 36 wt% hydrochloric acid was added dropwise at 25°C over 30 minutes. After the dropwise addition, the reaction solution was further stirred for 30 minutes. The resulting crude crystals were filtered, washed with 15 ml of ethyl acetate, and dried under reduced pressure at 50° C. for 2 hours to obtain 9.20 g (89%) of the target compound as a white powder.
[0109] 1 H-NMR spectrum (DMSO-d 6 , Δppm): 0.99 (d; J = 6Hz, 3H), 1.19 (dd; J = 6Hz, 3Hz, 3H), 2.29 (s, 3H), 4.03-4.27 (m, 4H), 4.73-4.90 (m, 2H), 4.93-5.31 (m, 1H), 5.68-6.00 (m, 1H), 6.94 (brs, 2H), 7.33-7.74 (m, 1...
Embodiment 2
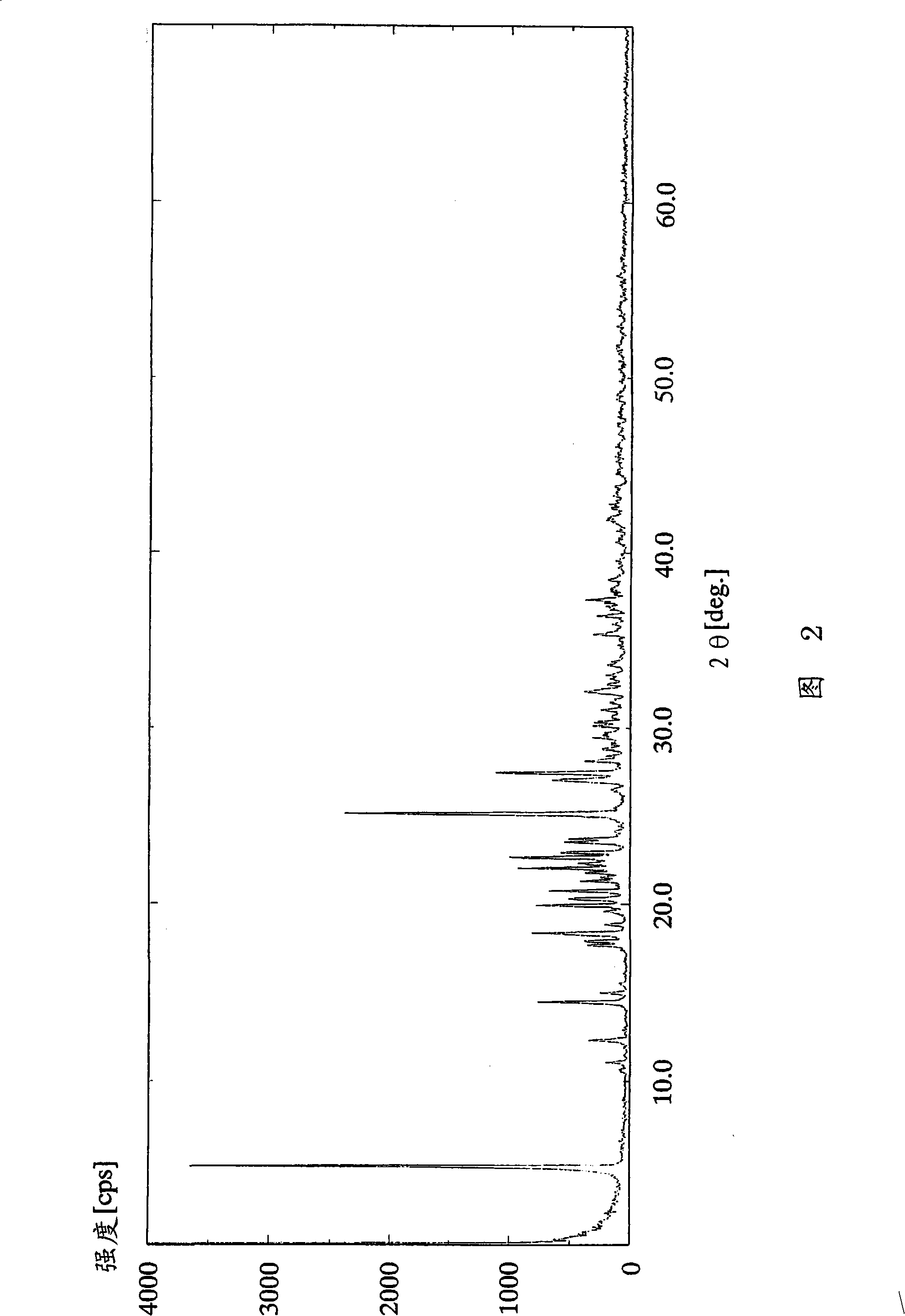
[0112] (Example 2) (R)-2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-di Phenylmethylazetidine-3-yl) ester 5-isopropyl ester dihydrobromide dihydrate
[0113] (2A) [Reaction solvent: acetone]
[0114] In (R)-2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-(1-diphenylmethyl To a solution of 2.91 g (5.00 mmol) of azetidine-3-yl) ester 5-isopropyl ester in 7 ml of acetone, 1.16 ml (10.0 mmol) of 47 wt% hydrobromic acid was added dropwise at 25°C over 30 minutes. After the dropwise addition, the reaction solution was further stirred for 30 minutes. The resulting crude crystals were filtered, washed with 7 ml of acetone, and dried at 60° C. under reduced pressure for 2 hours to obtain 2.68 g (69%) of the target compound as a white powder.
[0115] 1 H-NMR spectrum (DMSO-d 6 , Δppm): 0.99(d; J=6Hz, 3H), 1.19(d; J=6Hz, 3H), 2.29(s, 3H), 4.01-4.38(m, 4H), 4.75-4.89(m, 2H) , 4.96-5.23 (m, 1H), 5.78-6.08 (m, 1H), 6.85 (brs, 2...
preparation example 1
[0200] (Preparation Example 1) Capsules
[0201] The powders of the compound of Example (10.0 mg), lactose (168.7 mg), corn starch (70.0 mg) and magnesium stearate (1.3 mg) (250 mg in total) were mixed, and after passing through a 60-mesh sieve, the powder was placed in No. 2 As a capsule in gelatin capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com