Method for preparing strong alkali anion exchange resin with long spacer arm
An exchange resin, spacer arm technology, applied in anion exchange, ion exchange, chemical instruments and methods, etc., can solve the problems of cumbersome process, difficult to control, difficult operation, etc., and achieve broad application prospects, cost saving, and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
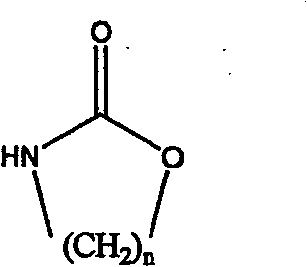
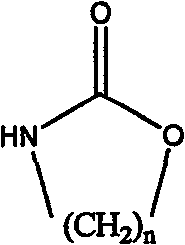
[0017] In a 250mL four-neck flask equipped with a stirrer, condenser and thermometer, add 15.9g (0.119mol) of triethylamine hydrochloride and 31.8g (0.238mol) of aluminum trichloride under nitrogen protection, and stir at 80°C for 2h , add 3g macroporous white spherical beads and 20ml nitrobenzene that have been swelled in nitrobenzene for 6h, then gradually add 12g (0.119mol) of 1,3-oxazin 2-ketone (a kind of cyclic carbamate), After reacting at 95°C for 24 hours, the aminopropylated white spherical beads were separated and extracted with ethanol for 2 hours to obtain aminopropylated copolymerized beads with a weak base exchange capacity of 2.21meq / g dry resin.
[0018] Weigh 3 g of the above-mentioned amine-propylated copolymerized beads, 10 mL of formaldehyde, and 15 mL of formic acid, react at 50° C. for 5 h, filter, extract, wash, and dry to obtain 3.6 g of tertiary-aminated copolymerized beads.
[0019] Add the copolymerized beads after tertiary amination and 10ml bromoe...
Embodiment 2
[0022] In a 250mL four-neck flask equipped with a stirrer, a condenser and a thermometer, add 100mL of nitrobenzene and 15.8g (0.119mol) of aluminum trichloride under nitrogen protection, start stirring, and after the aluminum trichloride is dissolved, add Swell 3g of gel beads in nitrobenzene for 6h, then gradually add 12g (0.119mol) of 1,3-oxazin-2-one, react at 80°C for 36h, and separate the aminopropylated white balls The beads were extracted with ethanol for 2 hours to obtain aminopropylated copolymerized beads with a weak base exchange capacity of 1.86meq / g dry resin.
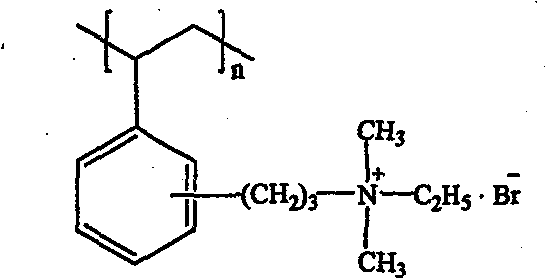
[0023] The tertiary amination and quaternization of Example 1 were used to obtain bromide quaternary ammonium anion exchange resins with an exchange capacity of 1.55meq / g. The structure is as follows:
[0024]
Embodiment 3
[0026] In a 250mL four-neck flask equipped with a stirrer, condenser and thermometer, add 31.3g (0.228mol) of triethylamine hydrochloride and 60.7g (0.455mol) of aluminum trichloride under nitrogen protection, and stir at 80°C for 2h , add 3g of macroporous white spherical beads swelled in fluorobenzene for 6h and 20ml of fluorobenzene, then gradually add 8g (0.091mol) of 2-oxazolidinone (a cyclic carbamate) and react at 120°C After 24 hours, the amine-ethylated white spherical beads were separated and extracted with ethanol for 2 hours to obtain amine-ethylated copolymerized beads, with a weak base exchange capacity of 1.96meq / g dry resin.
[0027] Tertiary amination and quaternization with Example 1 to obtain quaternary ammonium bromide strong base anion exchange resin with an exchange capacity of 1.75meq / g. The structure is as follows:
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com