Tablet formulations and processes
A technology for pharmaceutical preparations and medicaments, which is applied in the directions of pill delivery, block delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
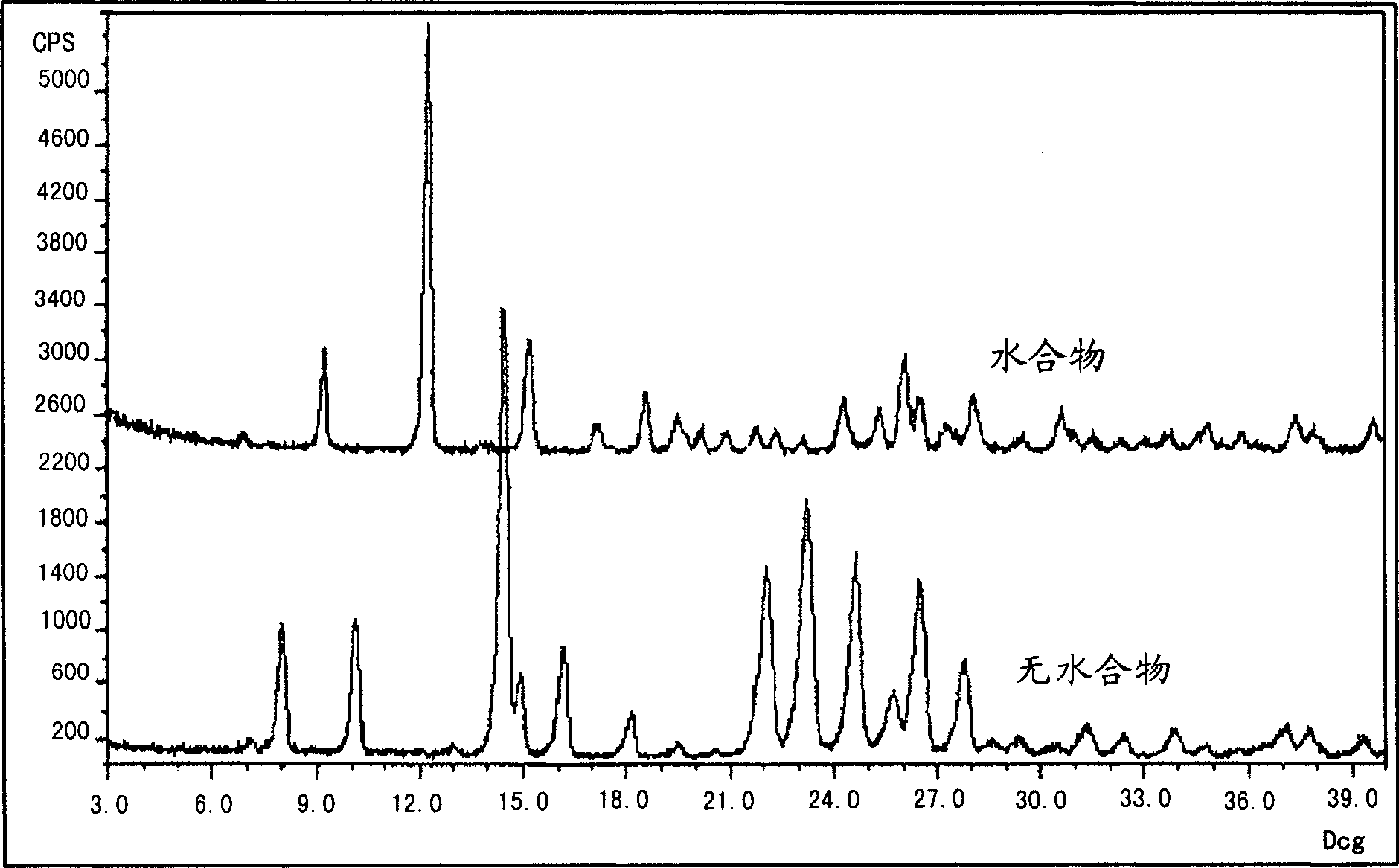
[0441] Preparation of anhydrate crystalline form of 2-(3-fluoro-4-hydroxyphenyl)-7-vinyl-1,3-benzoxazol-5-ol
[0442] Dissolve the solid of 2-(3-fluoro-4-hydroxyphenyl)-7-vinyl-1,3-benzoxazol-5-ol (170 g, 0.627 mol) in ethyl acetate at 75-80°C ester (3946 g, 23 vol). The resulting solution was treated with charcoal (17 g) at 75-80°C. The filtrate was then concentrated to 7 volumes at atmospheric pressure, heptane (793 g, 6 volumes) was added to the slurry maintained at 75-80 °C, then cooled to 45-50 °C and maintained for 0.5 hours, followed by cooling to 0-5 °C and maintained for 1 hour. The solids were filtered off and dried at 55-65°C and 5-10 mg Hg to give 87% recovery with a product purity of 99.4%.
Embodiment 2
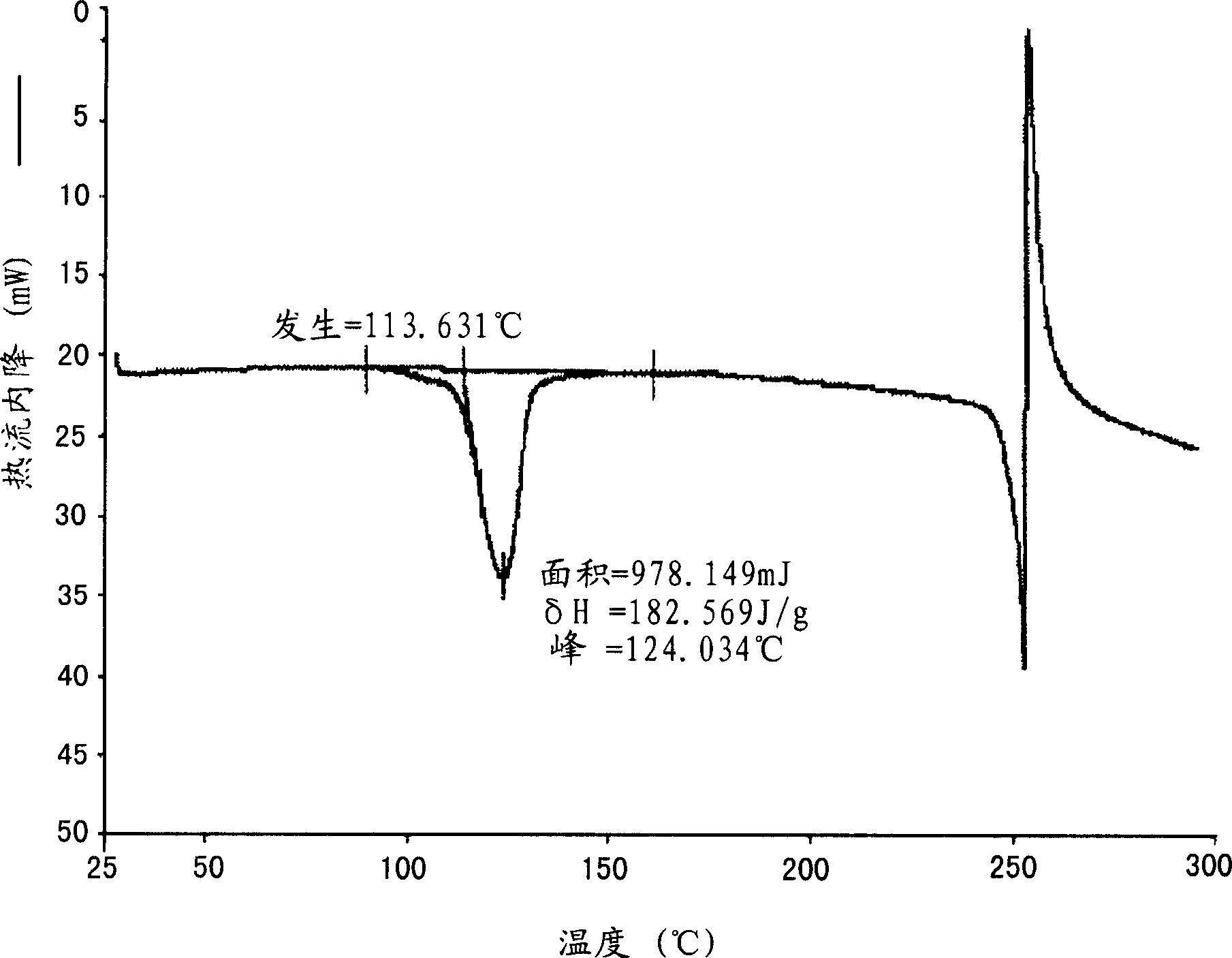
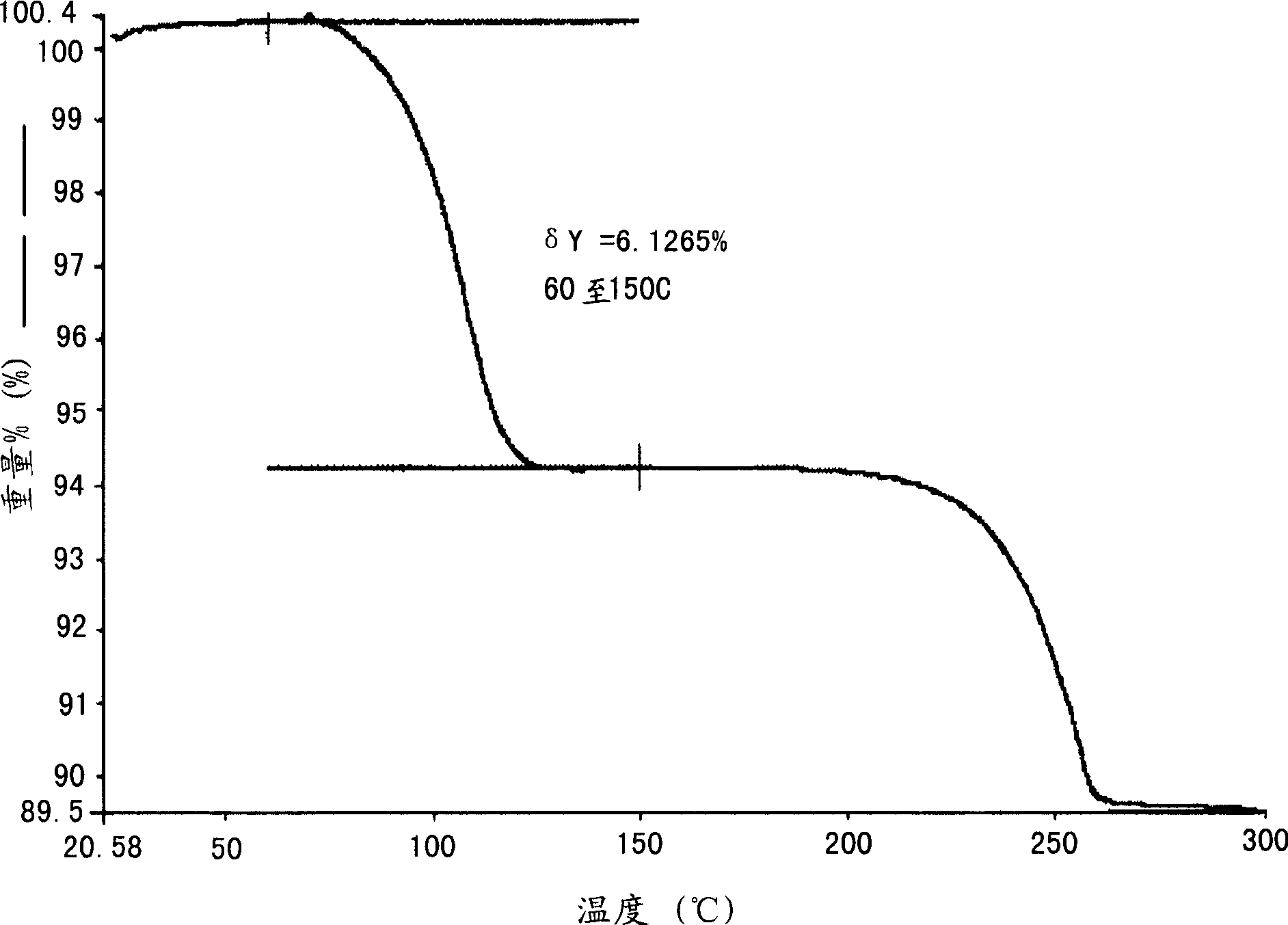
[0444]Preparation of monohydrate crystalline form of 2-(3-fluoro-4-hydroxyphenyl)-7-vinyl-1,3-benzoxazol-5-ol
[0445] Put 274 g of 2-(3-fluoro-4-hydroxyphenyl)-7-vinyl-1,3-benzoxazol-5-ol and 1375 ml of pre-filtered ethanol into a tank equipped with stir bar, condenser and temperature Probe inside a 3 L multi-neck flask. The mixture was heated to 75-80°C and a solution formed after 10 minutes. Water (688ml) was added to the solution during 0.5 hours at 75-80°C. The solution was cooled to 50°C over the course of 0.5 hours and then maintained at 50°C for 0.5 hours (crystallization started at about 74°C). The resulting suspension was then cooled to 0-5°C over the course of 0.5 hours and maintained at 0-5°C for 1 hour. The solid was collected by filtration and the filter cake was washed with 2 x 300 ml of ethanol:water (2:1 v / v) pre-cooled to 0-5°C. The washed filter cake was dried at 32-38°C and 20-25 mg Hg for 20 hours to yield 281.8 g (96.11% yield) of the final monohydrat...
Embodiment 3
[0447] Convert anhydrous to monohydrate crystalline form
[0448] pH method
[0449] Anhydrous 2-(3-fluoro-4-hydroxyphenyl)-7-vinyl-1,3-benzoxazol-5-ol (71 mg) was added to 2 ml of water and at the time the solution started to become clear The pH was adjusted to pH 10 with 1N NaOH. After 2 hours, the solution turned cloudy pale yellow. The solution was centrifuged, the supernatant was removed, and the precipitate was air dried and then vacuum dried. The XRPD and TGA of this product were consistent with the monohydrate.
[0450] Solvent / anti-solvent method
[0451] Dissolve anhydrous 2-(3-fluoro-4-hydroxyphenyl)-7-vinyl-1,3-benzoxazol-5-ol (about 100mg) in 3ml of ethanol, then slowly add 4ml of water until the solution becomes cloudy. The solution was centrifuged, the supernatant was removed, and the precipitate was air dried and then vacuum dried. The XRPD and TGA of this product were consistent with the monohydrate.
[0452] aqueous suspension method
[0453] Anhydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com