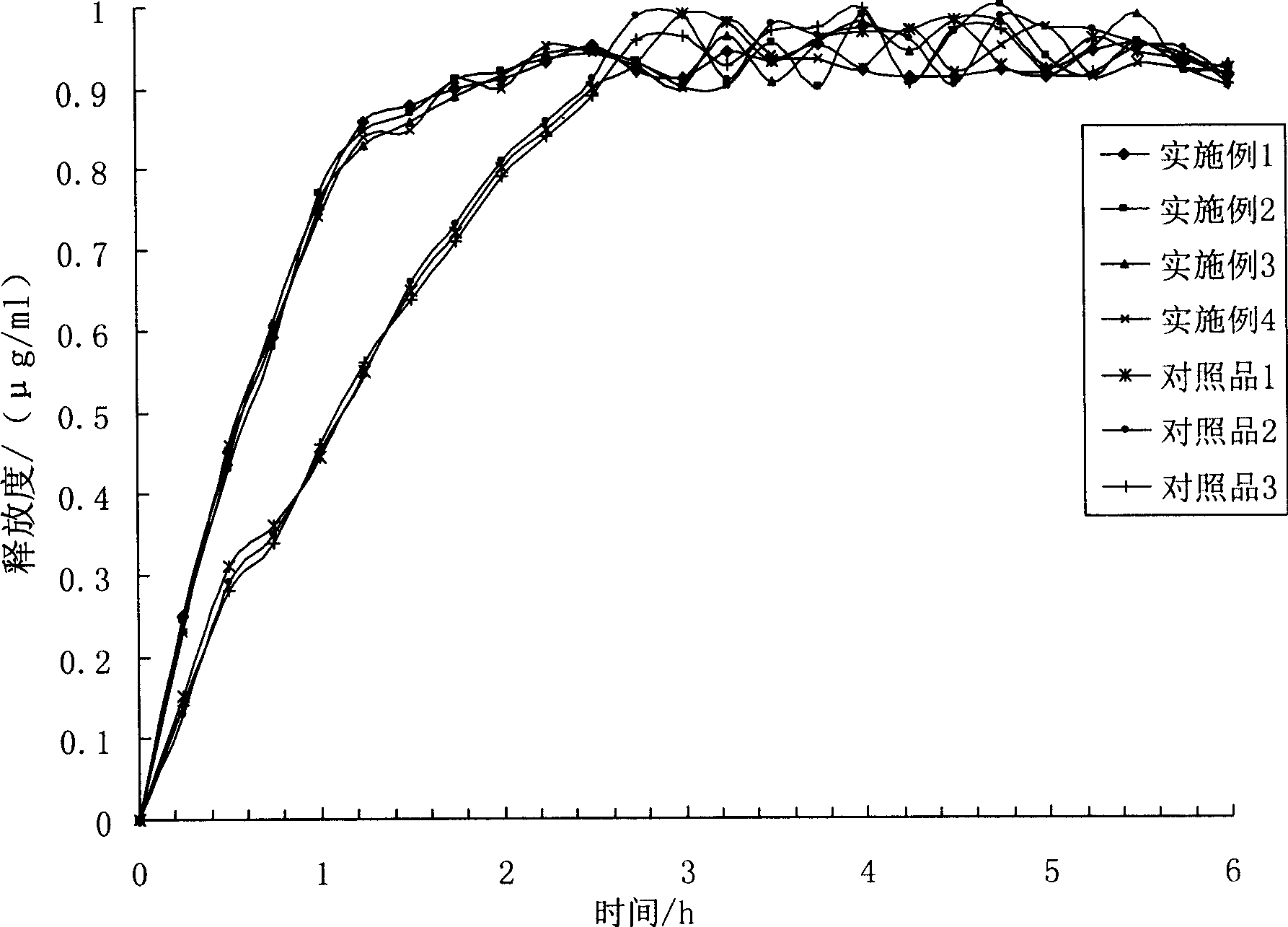
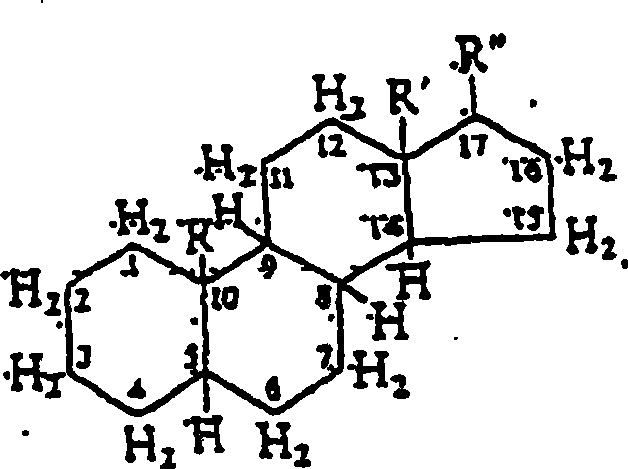
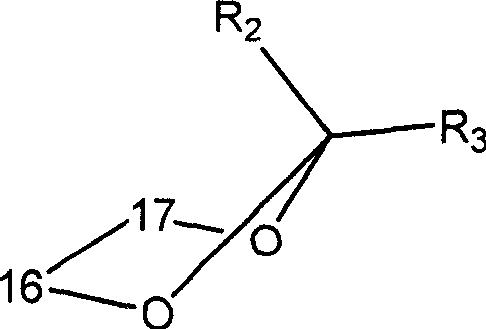
Nitric oxide-releasing glucocorticosteroid
A technology of corticosteroids and nitric oxide, applied in the field of anti-inflammatory drugs and NO-donating glucocorticoids, which can solve the problems of general anti-inflammatory effects of glucocorticoids and insignificant side effects of glucocorticoids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Synthesis of Furazamethylprednisolone
[0101] 1) Synthesis of 4-(methylprednisolone-21-oxo-)-4-oxo-butyric acid (3.1)
[0102] Dissolve 5.62g (15mmol) of methylprednisolone in 80ml of pyridine, add 3g (30mmol) of succinic anhydride, heat and reflux for 10 hours to stop the reaction, pour it into 200ml of saturated ice brine after cooling, adjust the pH to 5 with hydrochloric acid, and let it stand A white solid was precipitated, filtered and washed with water to obtain 6.01 g of the product with a yield of 84.4%.
[0103] 2) 4-(methylprednisolone-21-oxo-)-4-oxo-butyric acid-2-[2-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide- Synthesis of 4-oxygen-)ethoxy]ethyl ester (9.1)
[0104] Dissolve 0.6g (1mmol) of compound (3.1) in 20ml of anhydrous dichloromethane, add 1mmol of (8.1), 0.206g (1mmol) of DCC, and a few capsules of DMAP, and react at room temperature for 20h until white flocculents appear in the reaction solution. The product was formed, filtered and concentrate...
Embodiment 2
[0110] Furazan dexamethasone
[0111] 1) Synthesis of 2-(dexamethasone-21-oxo-)-2-oxo-acetic acid (3.2)
[0112] Dissolve 5.89g (15mmol) of dexamethasone in 80ml of pyridine, add 2.58g (30mmol) of oxalic anhydride, stop the reaction after heating back for 8 hours, pour it into 200ml of saturated ice-salt water after cooling, and adjust the pH to 5 with hydrochloric acid to stand still Precipitate a white solid, filter and wash with water to obtain 4.69g of product, yield 65.3% IR
[0113] 2) 2-(dexamethasone-21-oxo-)-2-oxo-acetic acid-2-[2-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4 - Oxygen -) ethoxy] ethyl ester (9.2) synthesis
[0114] Dissolve 0.5g (1mmol) of compound (3.2) in 20ml of anhydrous dichloromethane, add 1mmol of (8.1), 0.206g (1mmol) of DCC, several capsules of DMAP, and react at room temperature for 20h until white flocculents appear in the reaction liquid The product was formed, filtered, and concentrated by column chromatography ethyl acetate:petroleum ...
Embodiment 3
[0121] Furazancisonide
[0122] 1) Synthesis of 4-(desisobutyryl ciclesonide-21-oxy)-4-oxo-butyric acid (3.3)
[0123] Dissolve 7.62g (15mmol) of deisobutyryl-ciclesonide in 80ml of pyridine, add 3g (30mmol) of succinic anhydride, heat and reflux for 10 hours to stop the reaction, pour it into 200ml of saturated ice-water brine after cooling, and wash with hydrochloric acid Adjust the pH to 5, and a white solid precipitates out after standing still. After filtering and washing with water, 5.5 g of the product is obtained with a yield of 55%.
[0124] 2) 4-(Deisobutyryl ciclesonide-21-oxygen)-4-oxo-butanoic acid [2-(3-benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4 Synthesis of -oxygen-)ethoxy]ethyl ester (9.3)
[0125] Dissolve 0.7g (1mmol) of compound (3.3) in anhydrous dichloromethane, add (8.1) 1mmol, 0.206g (1mmol) of DCC, several capsules of DMAP, and react at room temperature for 20h until a white flocculent product is formed in the reaction solution , filtered, and concen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap