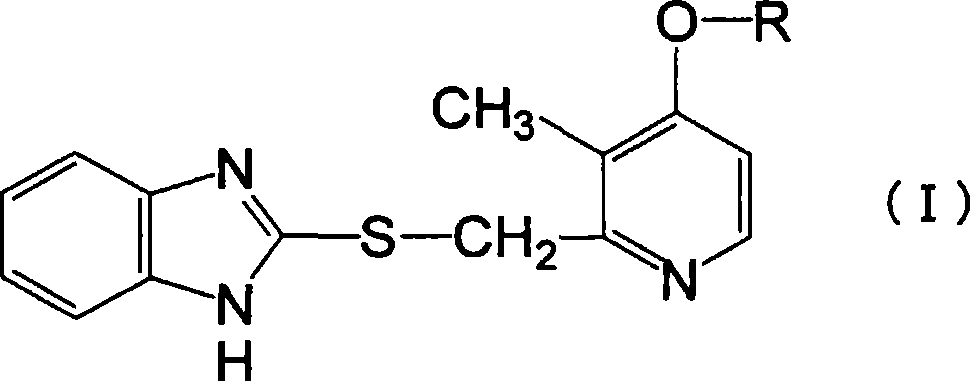
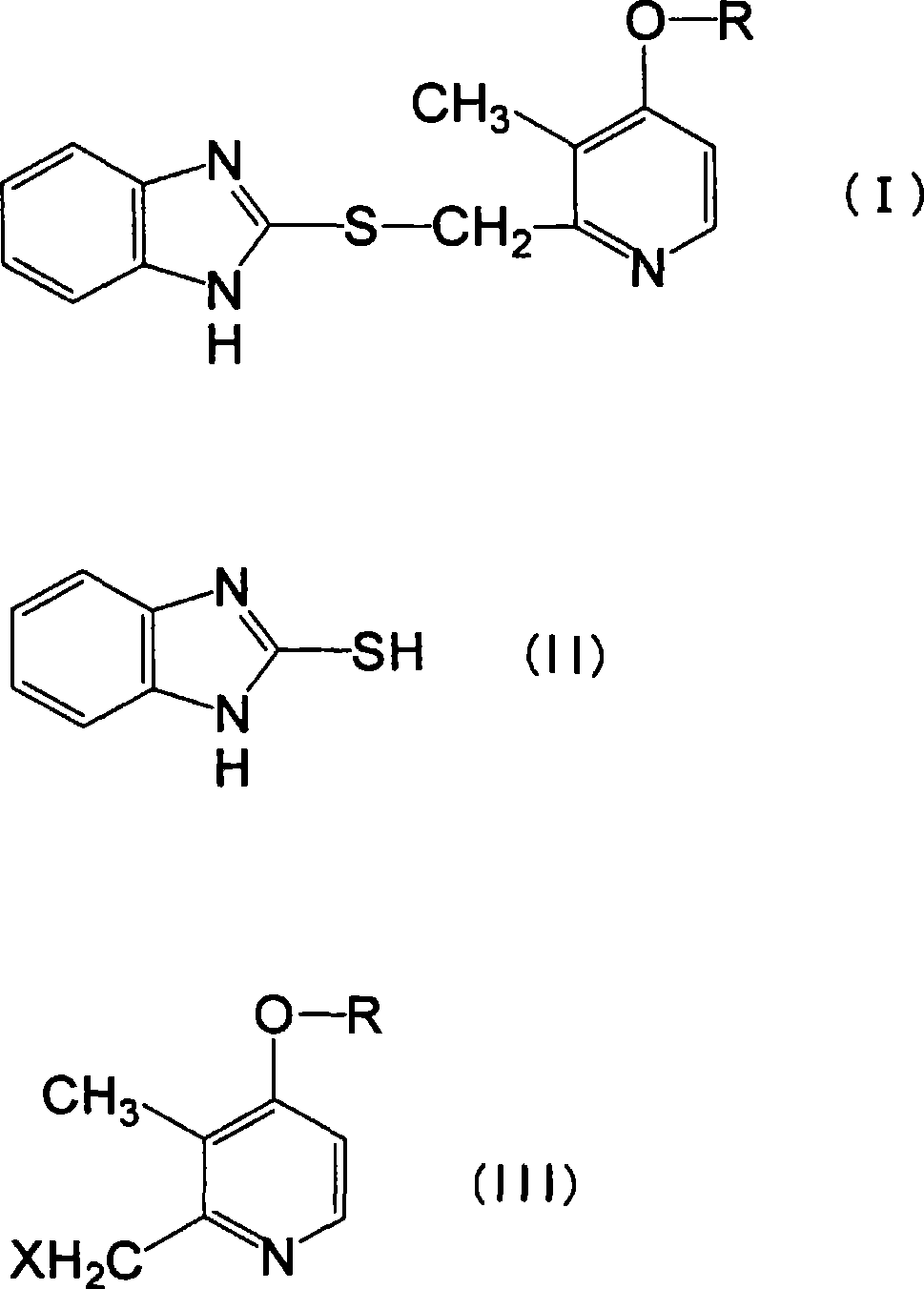
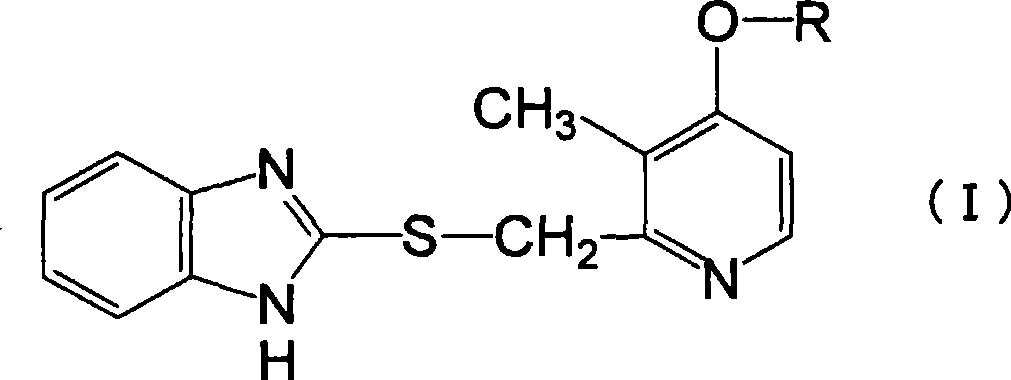
Novel pyridine derivative having anti-helicobacter pylori activity
An anti-Helicobacter pylori and derivative technology, applied in antibacterial drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. The record and enlightenment of bacterial action, etc., to achieve the effect of excellent antibacterial action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0076] Hereinafter, methods for producing raw material compounds used in the present invention, comparative compounds, and compounds of the present invention will be specifically described by way of Synthesis Examples and Examples. In addition, HPLC analysis was performed under the following conditions.
[0077] Column Inertsil ODS-3 150mm×4.6mmID
[0078] Eluent 0.05M KH 2 PO 4 / acetonitrile=50 / 50(v / v)
[0079] Flow rate 1.0ml / min
[0080] Column temperature 40°C
[0081] Injection volume 2μL
[0082] Detection wavelength 254nm
Synthetic example 1
[0083] Synthesis example 1: 4-(5-hydroxypentyloxy)-2,3-lutidine-N-oxide
[0084] Under nitrogen flow, 140 ml of 1,5-pentanediol was added on a silicone oil bath, and 4.6 g (0.2 mol, 2.0 equivalents) of sodium metal was added while stirring. Next, the silicone oil bath was heated and reacted at 100° C. for 1 hour. After adding 15.8 g (1.0 mol, 1.0 equivalent) of 4-chloro-2,3-lutidine-N-oxide to the obtained reaction liquid, the temperature was raised to 120° C. and reacted for 2 hours. After cooling the reaction solution, it was concentrated and dried under reduced pressure to obtain 53.3 g of a concentrated residue, which was purified through a silica gel column to obtain 25.0 g of 4-(5-hydroxypentyloxy)-2,3-lutidine-N-oxide things.
Synthetic example 2
[0085] Synthesis Example 2: 4-(5-Hydroxypentyloxy)-2-acetoxymethyl-3-methylpyridine
[0086] Add 153.1g (1.5mol, 15 equivalents) of acetic anhydride to 24.5g (0.1mol, 1.0 equivalents) of 4-(5-hydroxypentyloxy)-2,3-lutidine-N-oxide, at 100 °C for 5 hours. Acetic anhydride was distilled off to obtain a concentrated residue, which was purified through a silica gel column to obtain 12.1 g of 4-(5-hydroxypentyloxy)-2-acetoxymethyl-3-picoline (yield 39.2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com