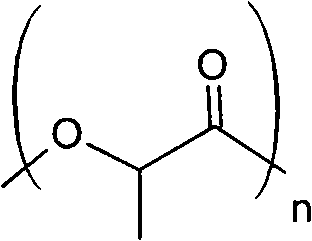
Subcutaneous implants containing a degradation-resistant polylactide polymer and a LH-RH analogue
一种LH-RH、植入物的技术,应用在聚交酯领域,能够解决不可控LH-RH类似物释放曲线等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] The mixture of polylactide and leuprolide acetate is weighed together, mixed uniformly by grinding, and then transferred to the extruder barrel of a plunger extruder.
[0108] Then, the mixture is heated to about 70°C to start the extrusion process. Then, the molten mixture is forced through the nozzle of the extruder by the force of the piston and cooled to ambient temperature. The resulting continuous beam is gradually cut into approximately 1 cm length (implant) and inserted into the application device (syringe). The syringe is initially packaged in an aluminum bag, sealed and sterilized by gamma sterilization with a dose between 25 and 32 kGy.
[0109] The weight average molecular weight of polylactide was measured before and after sterilization. The weight average molecular weight is measured by gel permeation chromatography (GPC). The measurement is carried out by a high-performance GPC device according to the German guide line for industry DIN55672 (German guide line...
Embodiment 2
[0113] In vitro release of leuprolide from implants
[0114] The dissolution characteristics of the implant were characterized by the following dissolution method. The principle of the described circulation device is based on the European Pharmacopoeia (Chapter 2.9.3., Dissolution test for solidoral dosage forms).
[0115] The implant is placed in the cylinder of the flow cell, which is closed with sintered filters at both ends. The medium (isotonic phosphate buffer (pH 7.4)) continuously flows through the chamber with the implant at a flow rate of approximately 0.3 ml / h. The entire pool is placed in a heated water bath at 37°C. An implant is allocated to a flow cell. The medium is collected at defined intervals and analyzed by a suitable HPLC method.
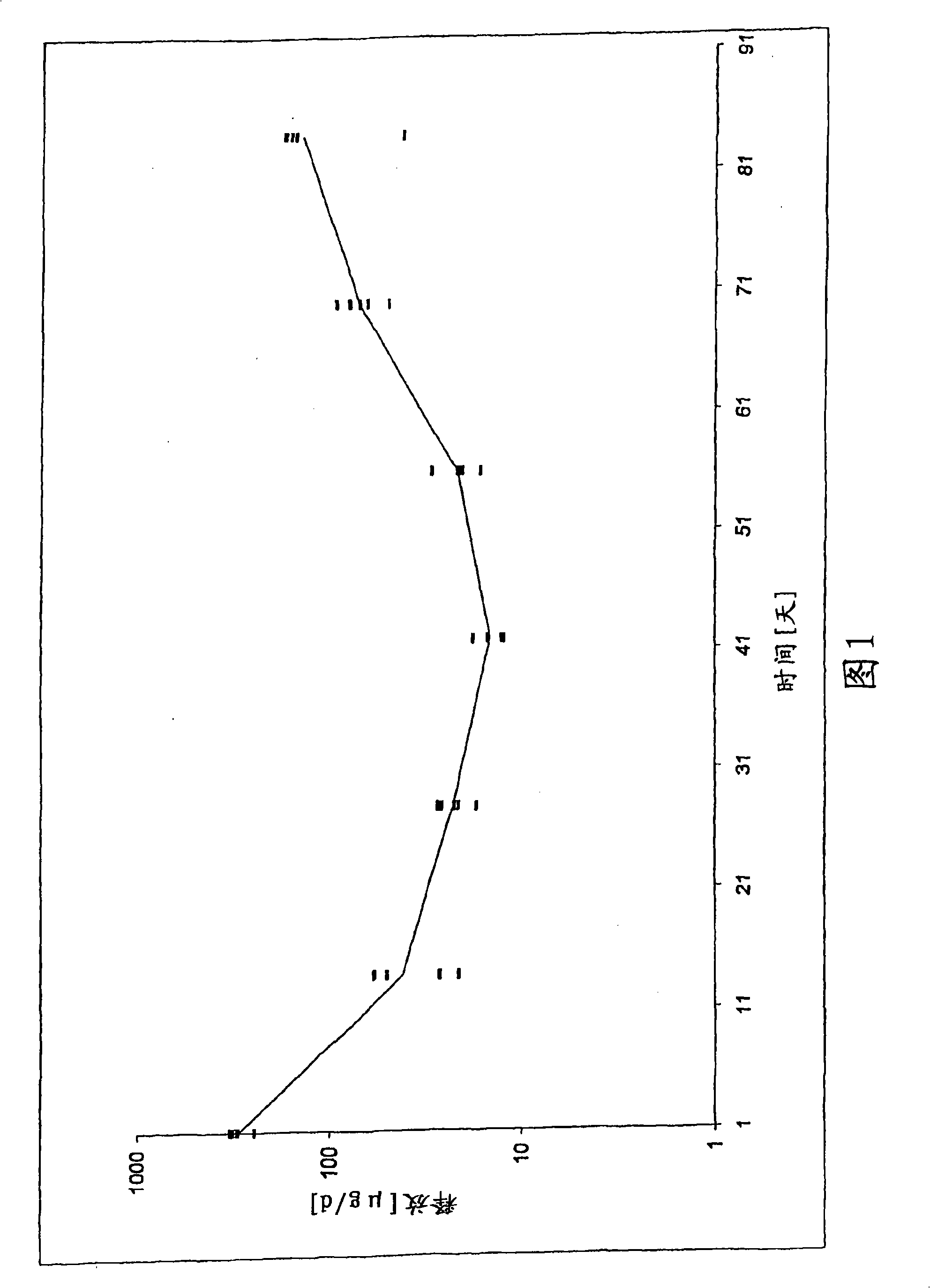
[0116] The in vitro release rates of leuprolide for the three batches of implants are shown in Table 2 and Figure 1.
[0117] Table 2: In vitro release of leuprolide from polylactide implants at 37°C.
[0118] Days
Embodiment 3
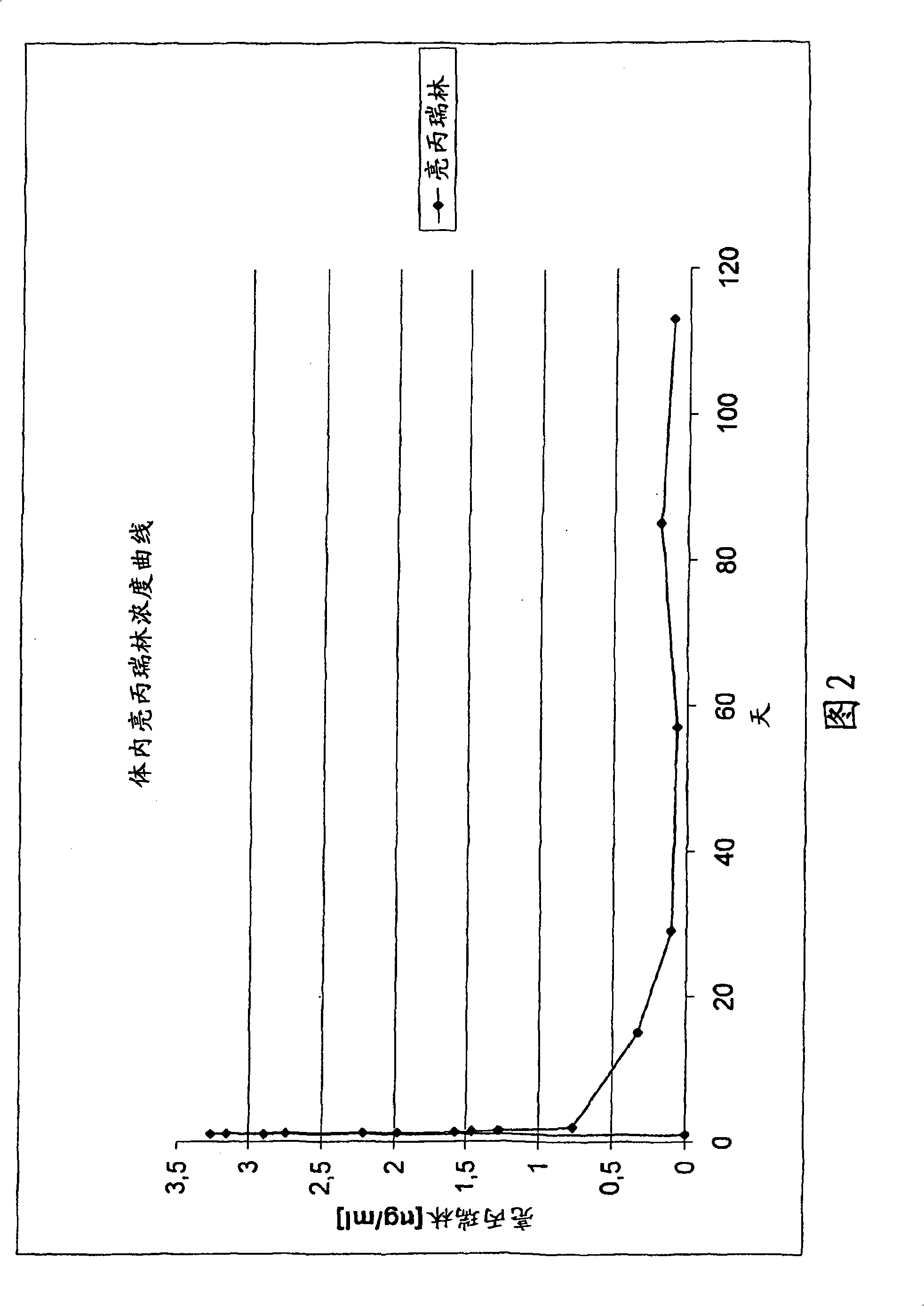
[0120] In vivo release of leuprolide implants
[0121] The implant composed of leuprolide acetate and polylactide was prepared according to Example 1. Based on leuprolide, the content by weight is 22.2w / w% of the implant. The weight average molecular weight of polylactide measured before sterilization was 7380 Daltons. The polydispersity index of polylactide is 1.51.
[0122] The pharmacokinetic in vivo test of this leuprolide implant was carried out with the following parameters:
[0123] Number of patients: 15
[0124] Method of administration: subcutaneous injection
[0125] Duration of treatment: 16 weeks (113 days)
[0126] Pharmacokinetics: Leuprolide was collected on days 1, 2, 15, 29, 57, 85, and 113.
[0127] The measurement of leuprolide in human serum samples is carried out by the following method:
[0128] -LC-MS / MS
[0129] -Calibration range: 25-10000pg / ml
[0130] -The lower limit of the quantity: 25pg / ml
[0131] The in vivo release of leuprolide from the implant i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com