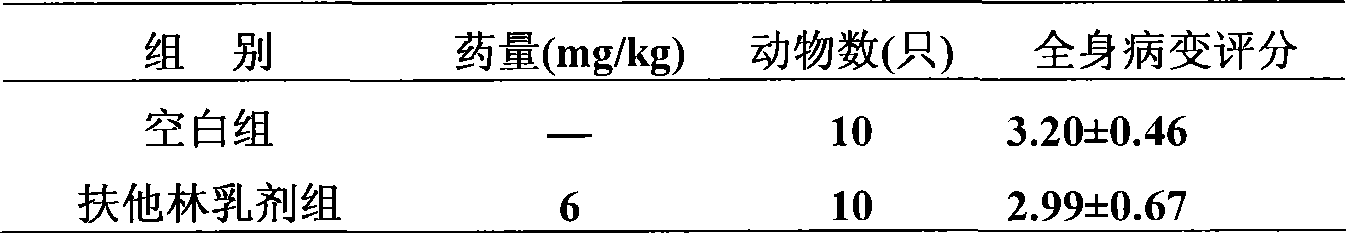External-use pharmaceutical composition formulation with antiphlogistic, swelling-dispersing and analgesic functions, and use
A technology of external drug and composition, applied in the field of external drug composition preparation, which can solve the problems of irritation, anti-inflammation, anti-swelling action time delay, shortening the use time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
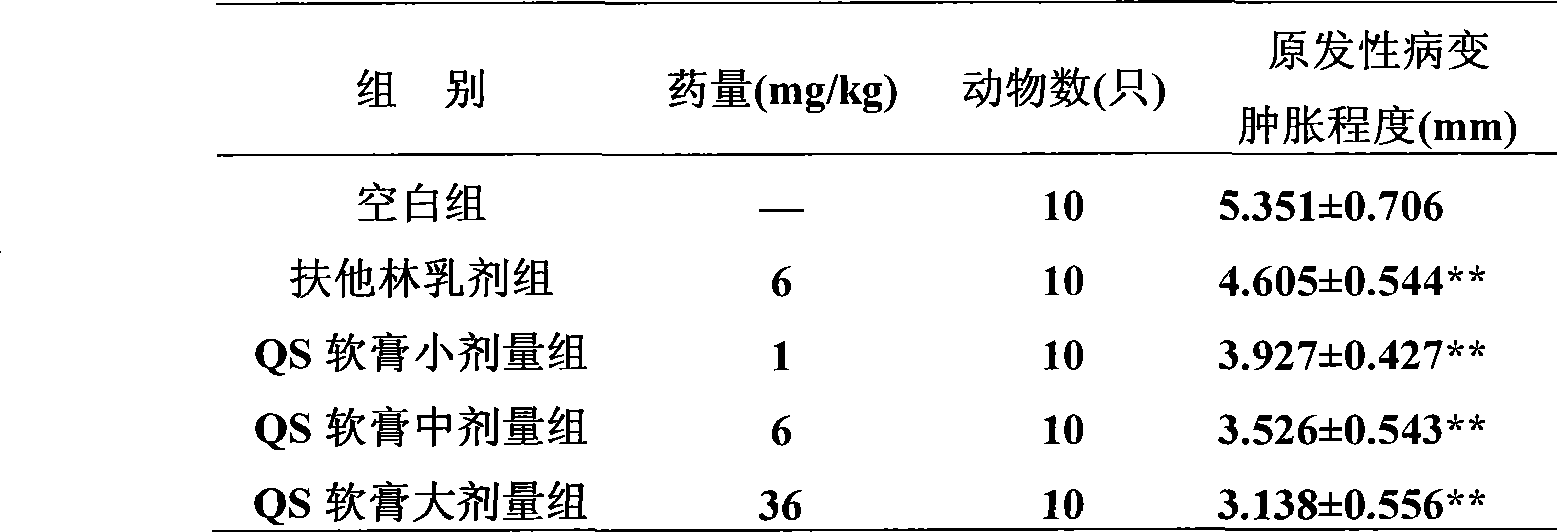
[0070] Example 1. An external pharmaceutical composition preparation with anti-inflammation, detumescence and analgesic effects, the active ingredient raw materials made into medicine and their weight percentages in the whole preparation are:
[0071] (1) Aescin or its pharmaceutically acceptable salt 0.01%;
[0072] (2) Diclofenac or its pharmaceutically acceptable salt 0.01%;
[0073] The rest are accessories.
Embodiment 2
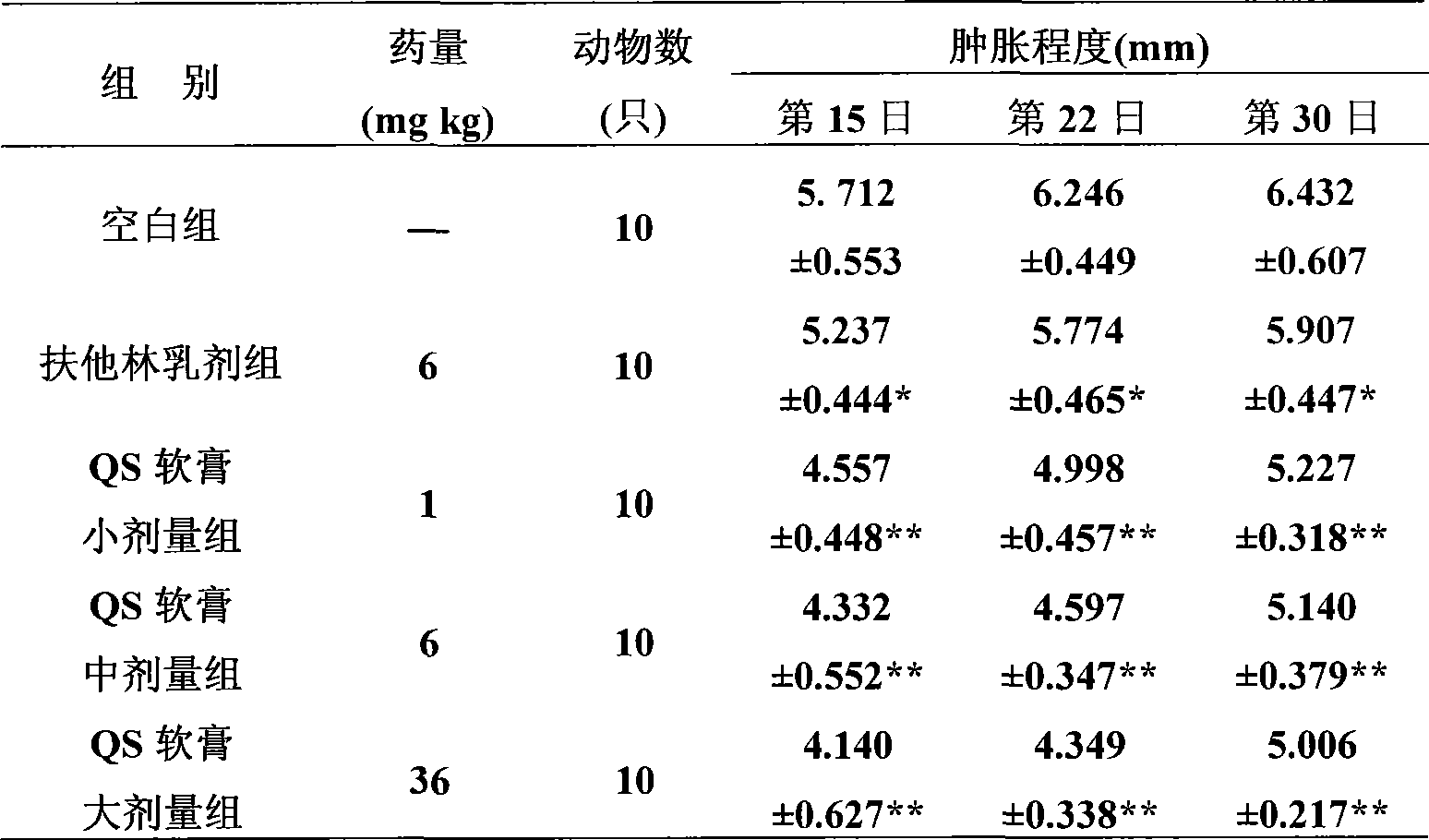
[0074] Example 2. An external pharmaceutical composition preparation with anti-inflammation, detumescence and analgesic effects, the active ingredient raw materials made into medicine and their weight percentages in the whole preparation are:
[0075] (1) Aescin and its pharmaceutically acceptable salt 20%;
[0076] (2) Diclofenac or its pharmaceutically acceptable salt 10%;
[0077] The rest are accessories.
Embodiment 3
[0078] Example 3. An external pharmaceutical composition preparation with anti-inflammation, detumescence and analgesic effects, the active ingredient raw materials made into medicine and their weight percentages in the whole preparation are:
[0079] (1) Aescin or its pharmaceutically acceptable salt 0.01%;
[0080] (2) Diclofenac or its pharmaceutically acceptable salt 10%;
[0081] The rest are accessories.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com