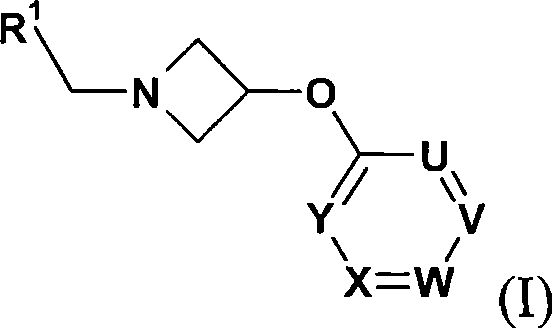
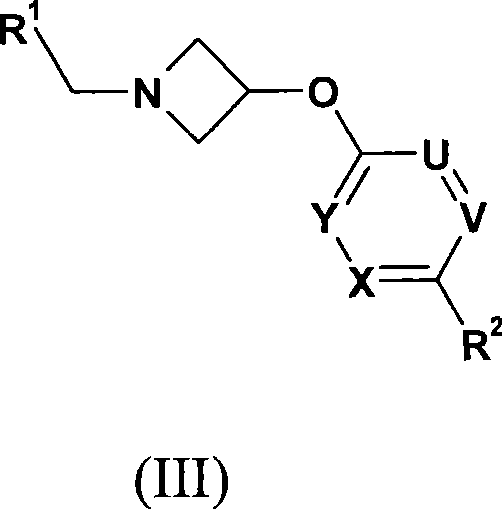
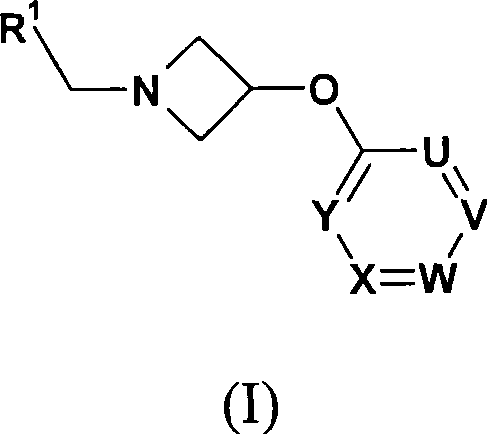
Azetidine derivatives as g-protein coupled receptor (GPR119) agonists
A technology of heterocyclic group and alkyl group, which is applied in the direction of drug combination, medical preparations containing active ingredients, metabolic diseases, etc., and can solve problems such as insufficient treatment of dyslipidemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0107] Further details on the preparation of compounds of formula (I) are found in the Examples.
[0108] Compounds of formula (I) can be prepared individually or as a compound library comprising at least 2, eg 5-1000 compounds of formula (I), more preferably 10-100 compounds of formula (I). Compound libraries can be prepared by combinatorial "split and mix" methods or multiple parallel syntheses using solution or solid phase chemistry using methods known to those skilled in the art.
[0109] In the synthesis of compounds of formula (I), labile functional groups such as hydroxyl, carboxyl and amino groups in intermediate compounds may be protected. Protecting groups may be removed at any step in the synthesis of compounds of formula (I), or present on the final compound of formula (I). An extensive discussion of the methods by which various labile functional groups can be protected and the methods for cleaving the resulting protected derivatives can be found, for example, in ...
Embodiment
[0157] LCMS protocol:
[0158] Waters Xterra MS C18, 5 μm (4.6 x 50 mm, flow rate 1.5 mL / min), in 12 min with 0.1% (v / v) ammonium in H 2 O-MeCN gradient elution with UV detection at 215 and 254 nm. Gradient information: 0.0-8.0min: from 95% H 2 O-5% MeCN up to 5% H 2 O-95% MeCN; 8.0-9.9min: keep at 5% H 2 O-95% MeCN; 9.9-10.0min: back to 95% H 2 O-5% MeCN; 10.0-12.0min: keep at 95% H 2 O-5% MeCN. Using positive ion (ESI + ) or anion (ESI - ) mode electrospray ionization source to obtain mass spectra.
[0159] Preparative LCMS protocol:
[0160] The gradient used for mass spectrometry-guided HPLC purification was as follows: Waters Xterra MSC18, 5 μm (19 x 50 mm, flow rate 25 mL / min) in 10 min with 0.1% (vol / vol) ammonium in H 2 O-MeCN gradient elution with UV detection at 215 and 254 nm. 0.0-0.50min: keep at 95%H 2 O-5% MeCN; 0.5-7.5min: from 95% H 2 O-5% MeCN up to 5% H 2 O-95% MeCN; 7.5-8.4min: keep at 5% H 2 O-95% MeCN; 8.4-8.5min: back to 95% H 2 O-5% MeCN;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com