Treating obesity with muscarinic receptor m1 antagonists
A technology for drugs and antidepressants, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., to solve problems such as failure to treat mental illness and failure of pirenzepine to produce behavioral effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
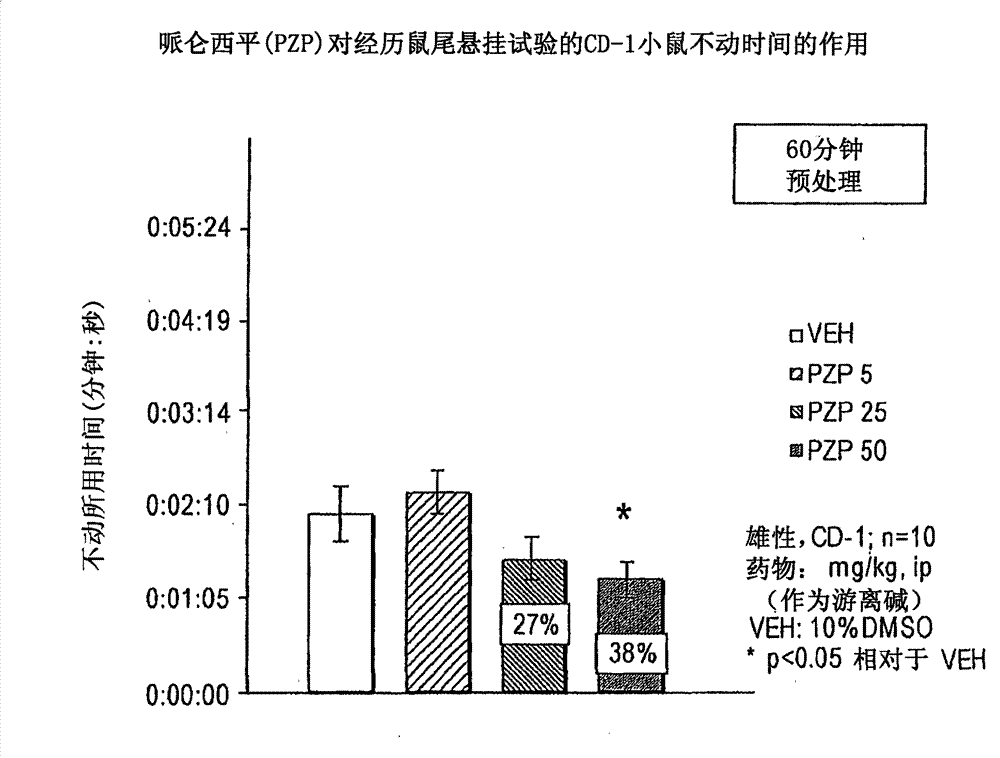
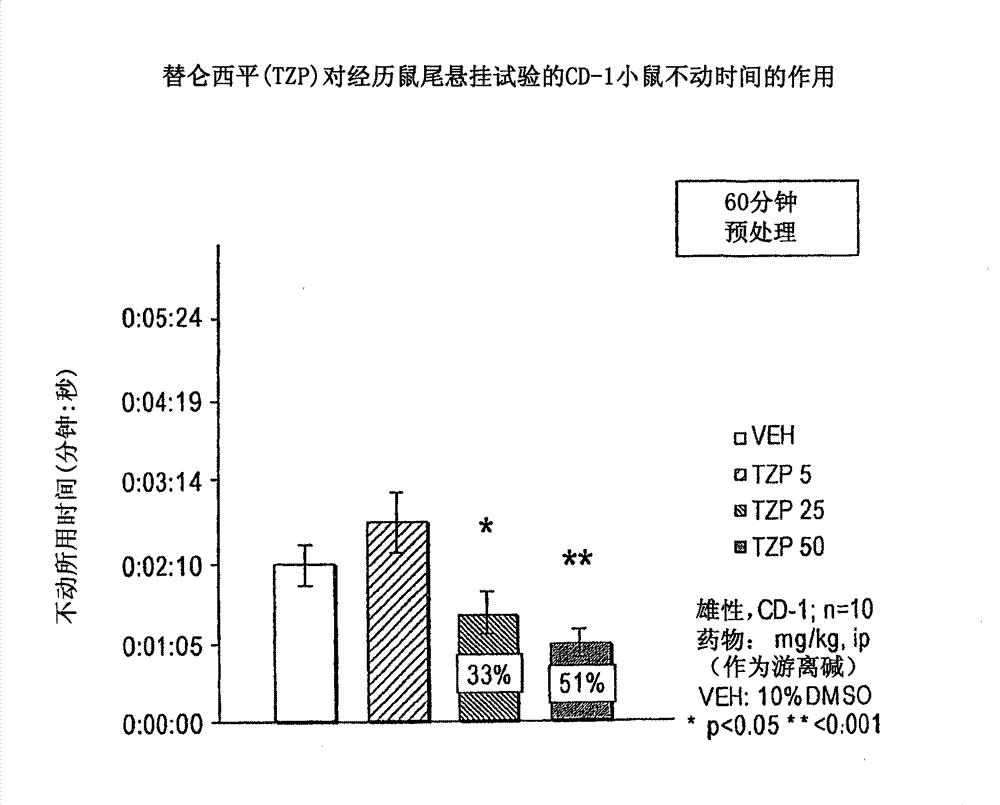
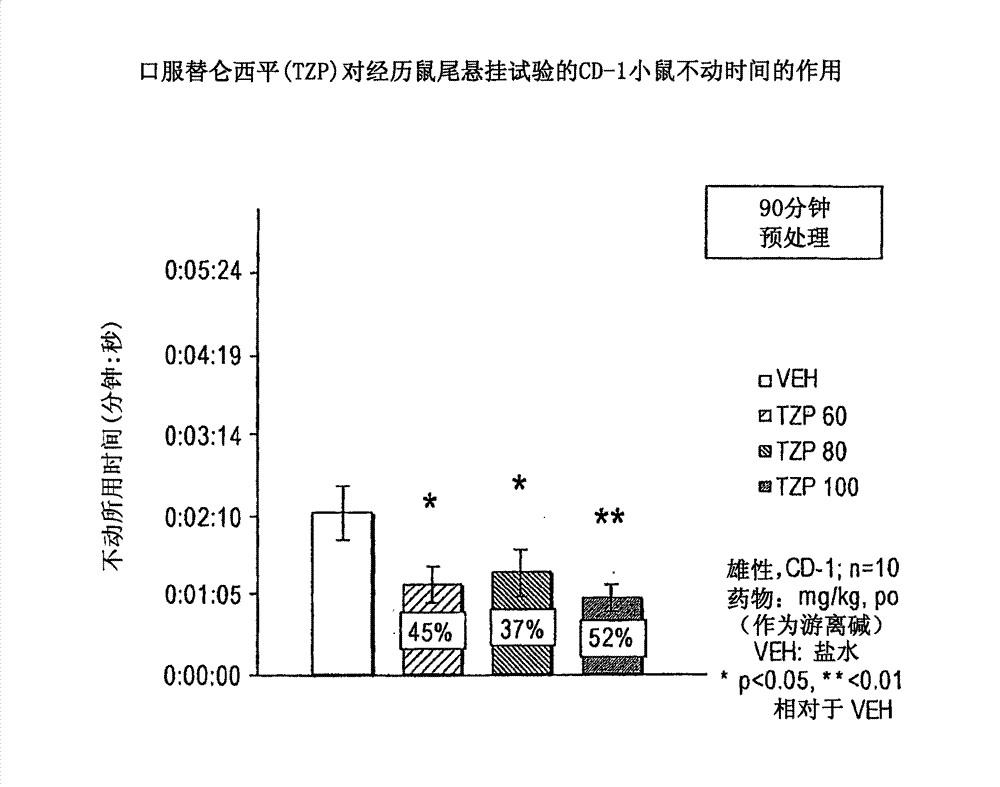
[0154] Antidepressant effect: The antidepressant effect of compounds was evaluated using the rat tail suspension method. The method was described by Steru et al., (Stem L, et al., Psychopharmacol 85:367-70, 1985; Stem L, et al., Prog Neuro-Psychopharmacol & Biol Psychiat 11:659-71, 1987) and later by Crowley et al. A variant of the human method (Crowley JJ, et al., Pharmacol Biochem Beh 78(2):269-74, 2004) was adapted. Seven to eight week old (25-35 g) male CD-1 mice were housed for one week prior to testing. Mice (n=8-10 / dose group) were dosed intraperitoneally (ip) or orally (po) with the drug under study and returned to their cages for appropriate pretreatment intervals (45- 60 minutes). Using adhesive tape, suspend the mouse from the tensiometer by its tail. Based on the intensity of movement recorded by the tensiometer, activity in the following 6 minutes was scored by computer: 1) immobility, 2) avoidance behavior or 3) severe avoidance behavior. Total immobility is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com