Halogenated furan ketone compound, and use thereof in antiinfective drug preparation
A technology of halogenated furanones and compounds, which is applied to the application field of organic compounds in biomedicine, can solve the problems of reduced antibacterial efficacy of antibiotics, drug resistance, difficulty in clinical treatment, etc., and achieves inhibition of bacterial biofilm formation, simple manufacturing process, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
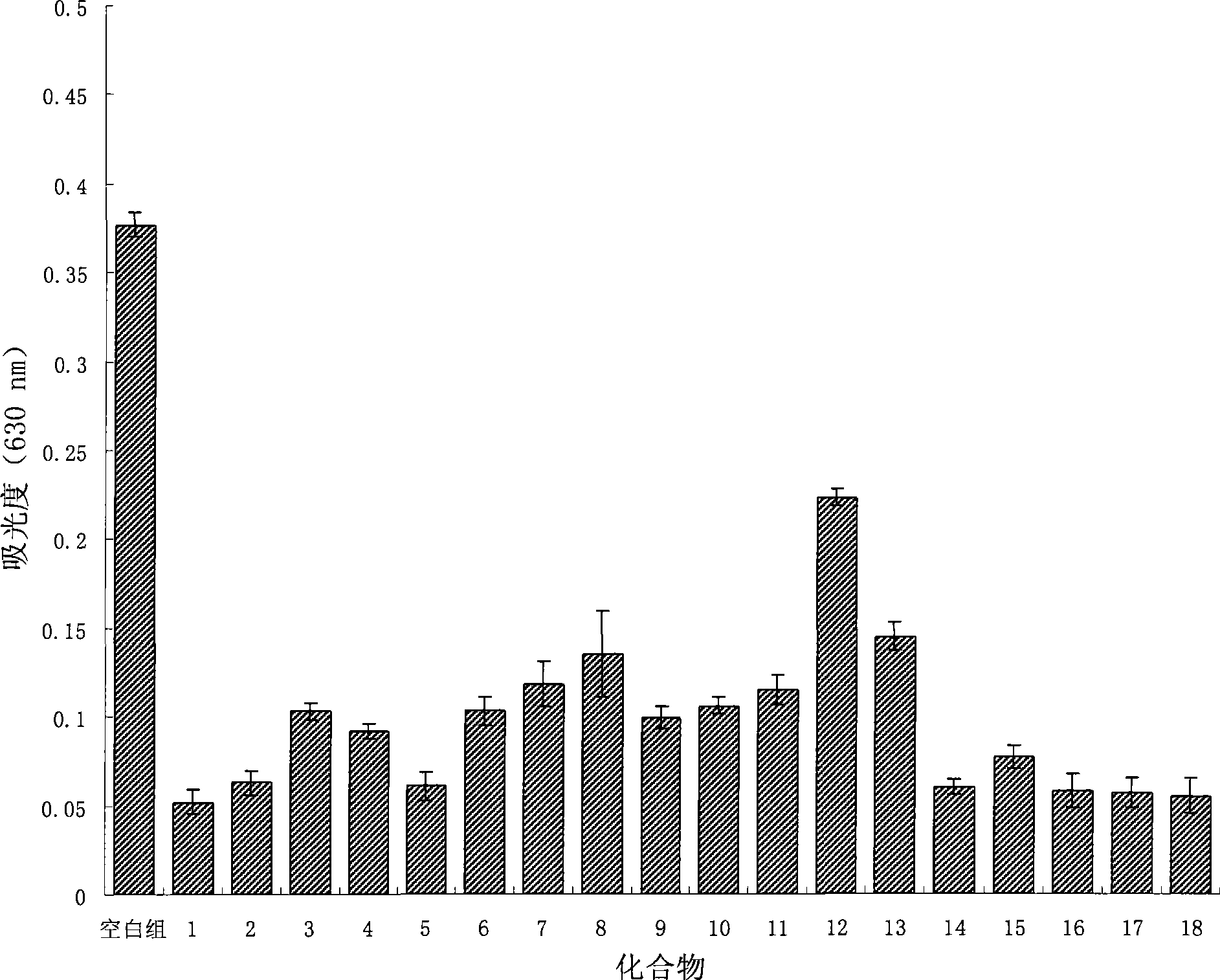
Image
Examples
Embodiment 1
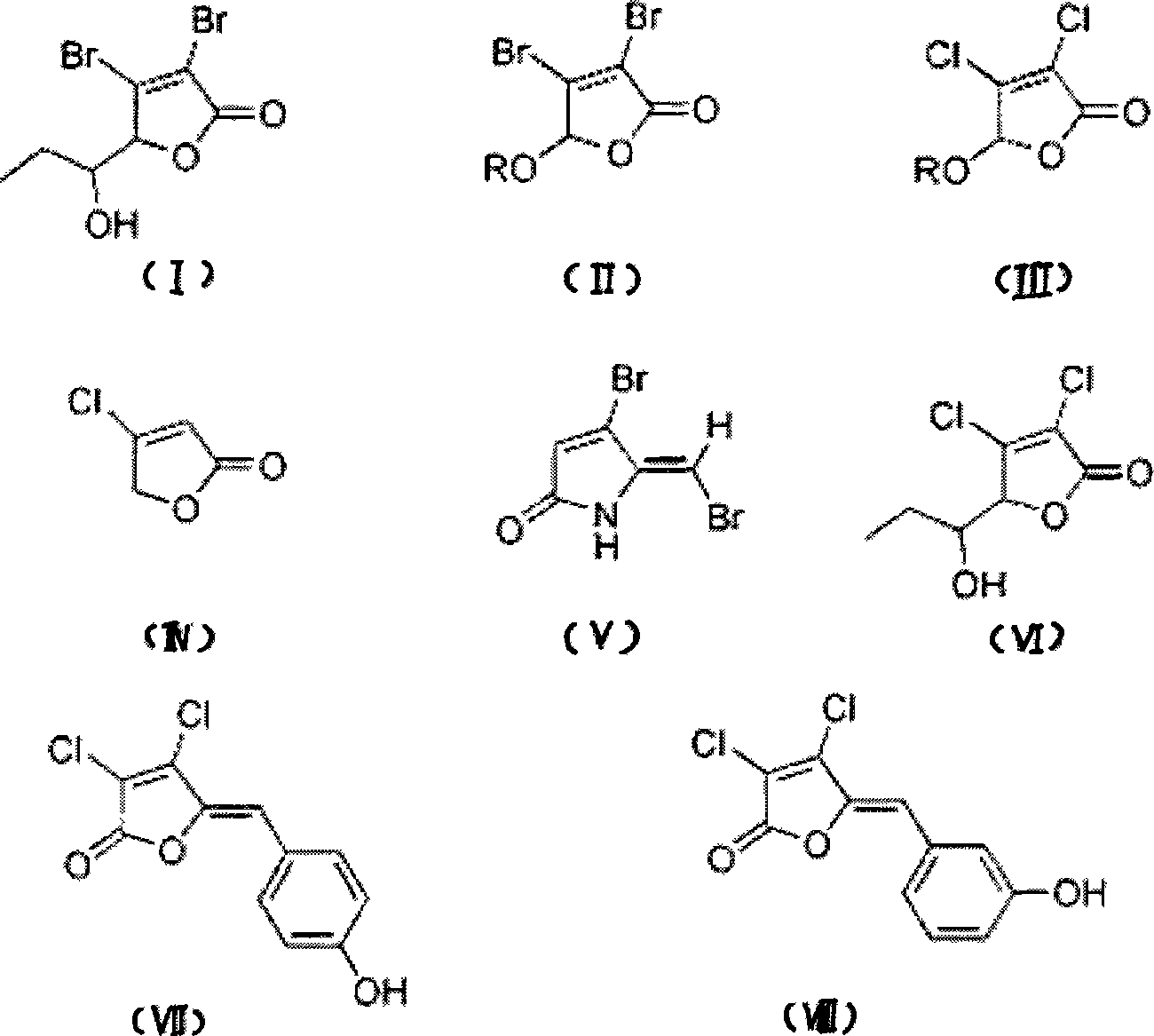
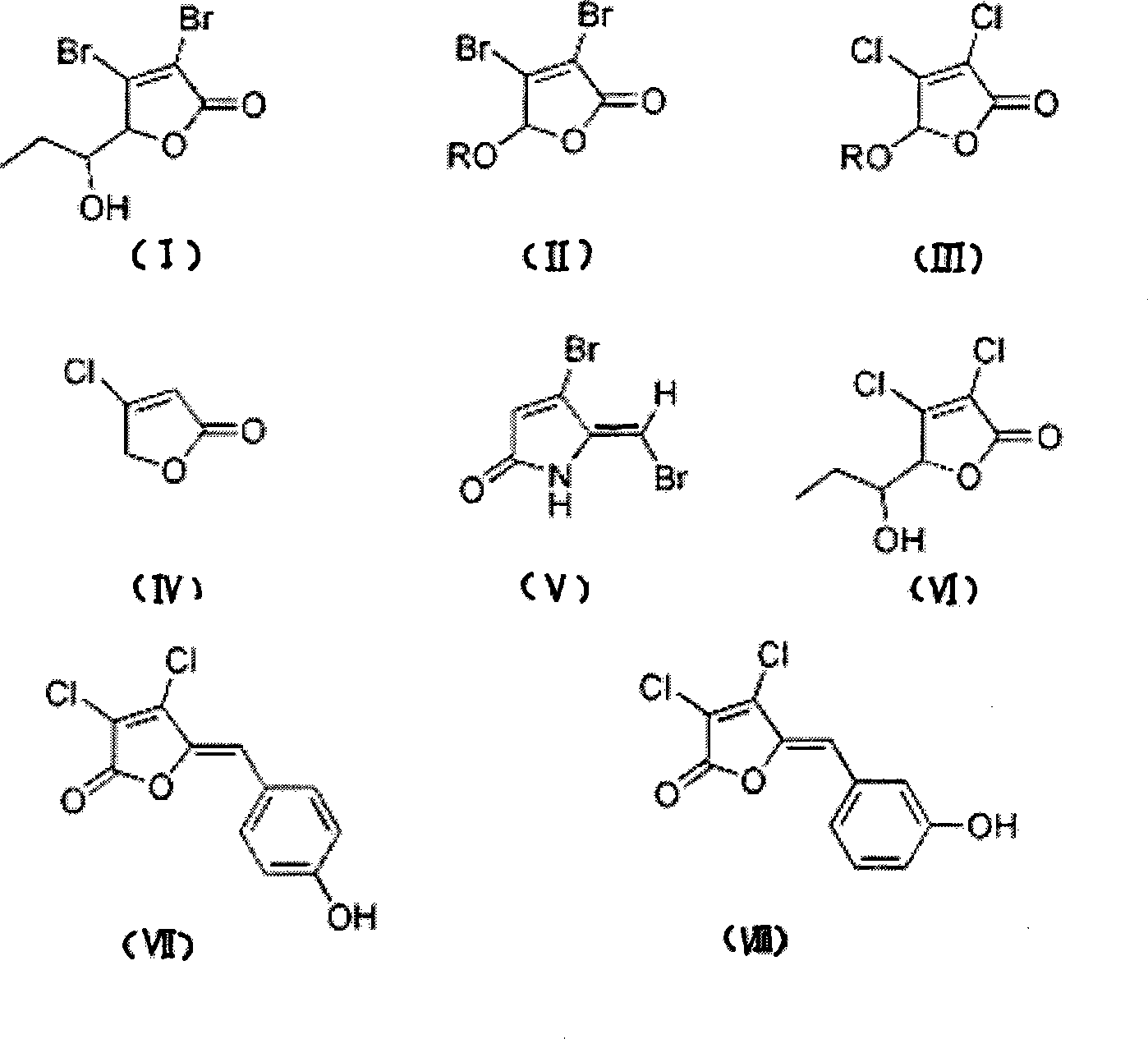
[0042] Synthesis of halogenated furanone compound shown in embodiment 1 formula (I)
[0043] Synthesis of this embodiment figure 1 The halogenated furanone compound shown in Chinese formula (I), its synthesis method adopts the chemical synthesis method of this field routine, specifically comprises the following steps:
[0044] (1) intermediate product-synthesis of halogenated furanone compound shown in formula (IX)
[0045] Add 9.6g of freshly steamed furfural and 110mL of water, add 86g of bromine under ice-bath cooling, raise the temperature and reflux for 0.5h, and concentrate under reduced pressure to obtain a light yellow solid; decolorize and recrystallize the light yellow solid with activated carbon to obtain a crude product, which is purified by silica gel column chromatography (V 乙酸乙酯 :V 石油醚 =1:6) to obtain 16.79 g of white solid, which is the compound represented by formula (IX), the Chinese name is 5-hydroxy-3,4-dibromo-2(5H)-furanone, and the yield is 65.1%.
...
Embodiment 2
[0054] Synthesis of halogenated furanone compounds shown in embodiment 2 formula (II)
[0055] Synthesis of this embodiment figure 1 The halogenated furanone compound shown in Chinese formula (II), its synthesis method adopts the conventional chemical synthesis method in this field, specifically comprises the following steps:
[0056] (1) Intermediate product—synthesis of compound shown in formula (IX)
[0057] The synthesis steps of the compound shown in formula (IX) are the same as in Example 1.
[0058] (2) When R=Me, the synthesis of final product 3,4-dibromo-5-methoxy-2(5H)-furanone
[0059] Dissolve 6.45g of the compound represented by the above formula (IX) in 11mL of methanol, add 1mL of concentrated sulfuric acid, reflux for 5h; cool to room temperature, add 60mL of water to quench the reaction, extract with benzene (60mL×3); then use 120mL of saturated Washed with sodium bicarbonate solution, dried over anhydrous sodium sulfate, filtered, and concentrated under re...
Embodiment 3
[0067] Synthesis of halogenated furanone compound shown in embodiment 3 formula (III)
[0068] Synthesis of this embodiment figure 1 The halogenated furanone compound shown in Chinese formula (III), its synthesis method adopts the chemical synthesis method conventional in this field, specifically comprises the following steps:
[0069] (1) When R=Me, the synthesis of 3,4-dichloro-5-methoxy-2(5H)-furanone
[0070] 4.23g of mucic acid was dissolved in 11mL of methanol, 1mL of concentrated sulfuric acid was added, and the reaction was refluxed for 5h; the follow-up treatment was the same as the operation when R=Me in Example 2, and finally 2.36g of colorless liquid was obtained, with a yield of 51.6%. The colorless liquid is 3,4-dichloro-5-methoxy-2(5H)-furanone, numbered as compound 9.
[0071] 1 H NMR (300MHz, CDCl 3 )δ 3.60(s, 3H), 5.78(s, 1H); MS(ESI): m / z 183(M+H) + , 205.1(M+Na) + .
[0072] (2) When R=Et, the synthesis of 3,4-dichloro-5-ethoxyl-2(5H)-furanone
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com