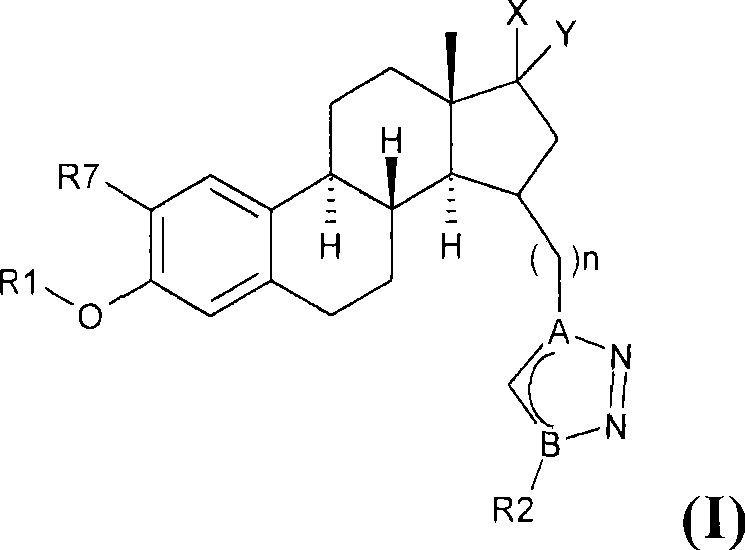
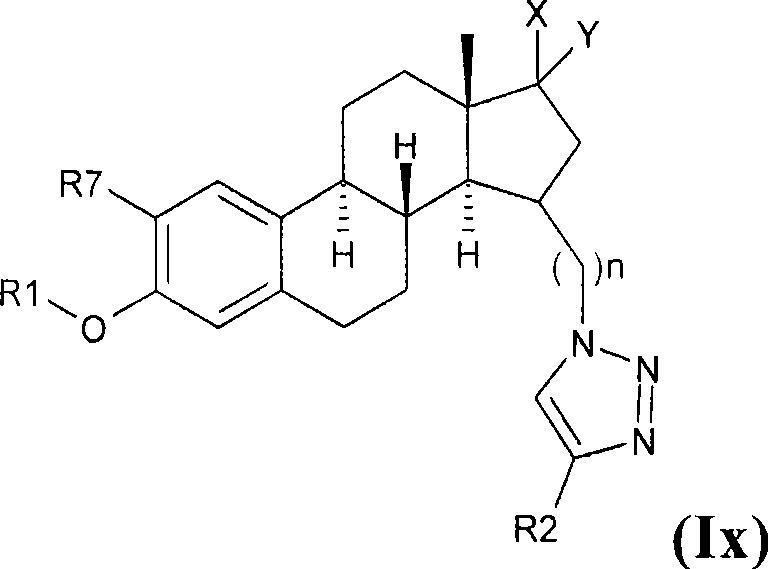
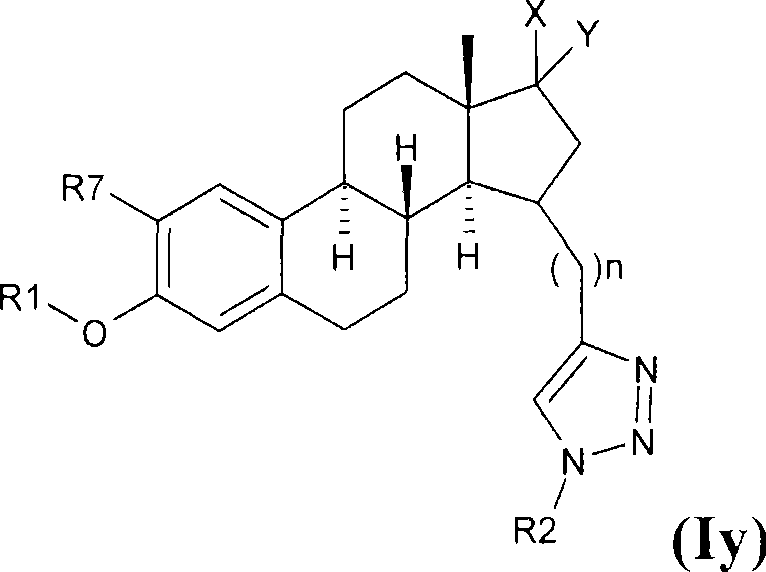
Estratriene derivatives and their uses as 17beta-hydroxysteroid dehydrogenase inhibitors
A technology based on alkyl and nitrile groups, applied in the field of new estratriene-triazole derivatives, can solve the problems of not being able to reach the level of T castration in men
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0272] definition
[0273] The following terms are used to describe the various components of the chemical compositions used in the present invention. The terms are defined as follows:
[0274] As used herein, the terms "comprises" and "including" are used herein in their open-ended, non-limiting sense.
[0275] The term "compound" is to be understood herein to encompass any and all isomers (such as enantiomers, stereoisomers, diastereomers, rotamers and tautomers), racemates or any mixture of isomers, prodrugs and any pharmaceutically acceptable salts of said compounds, unless the formula describing the compound clearly shows a particular stereochemistry.
[0276] When the plural form is used for compounds, salts, etc., it is also used to refer to a single compound, salt, etc.
[0277] The term "17β-hydroxysteroid dehydrogenase type I" or simply "17β-HSD1" is used for the enzyme EC 1.1.1.62, and reduces estrone (E1) to the biologically active estrogen, estradiol (E2) .
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com