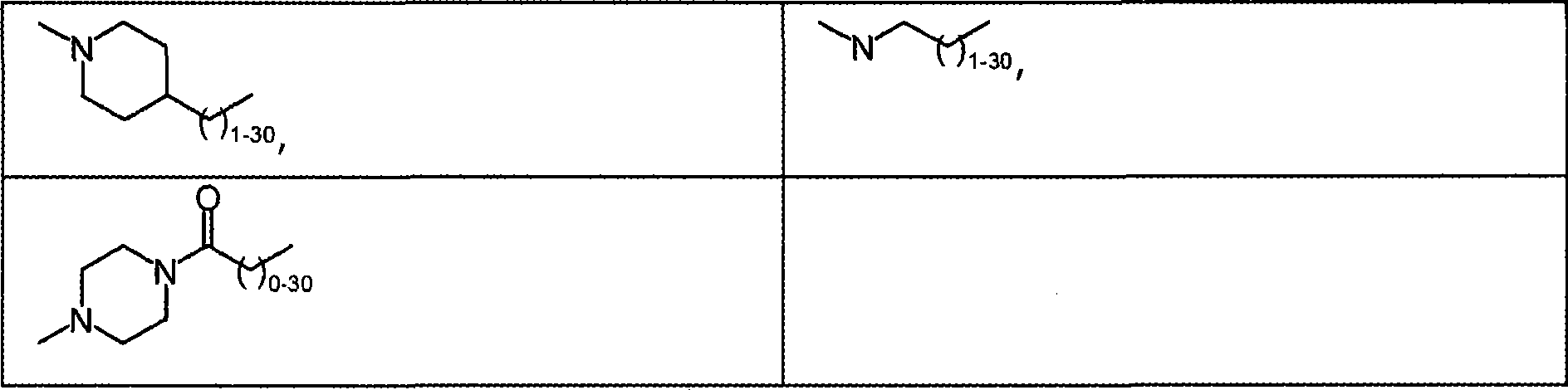
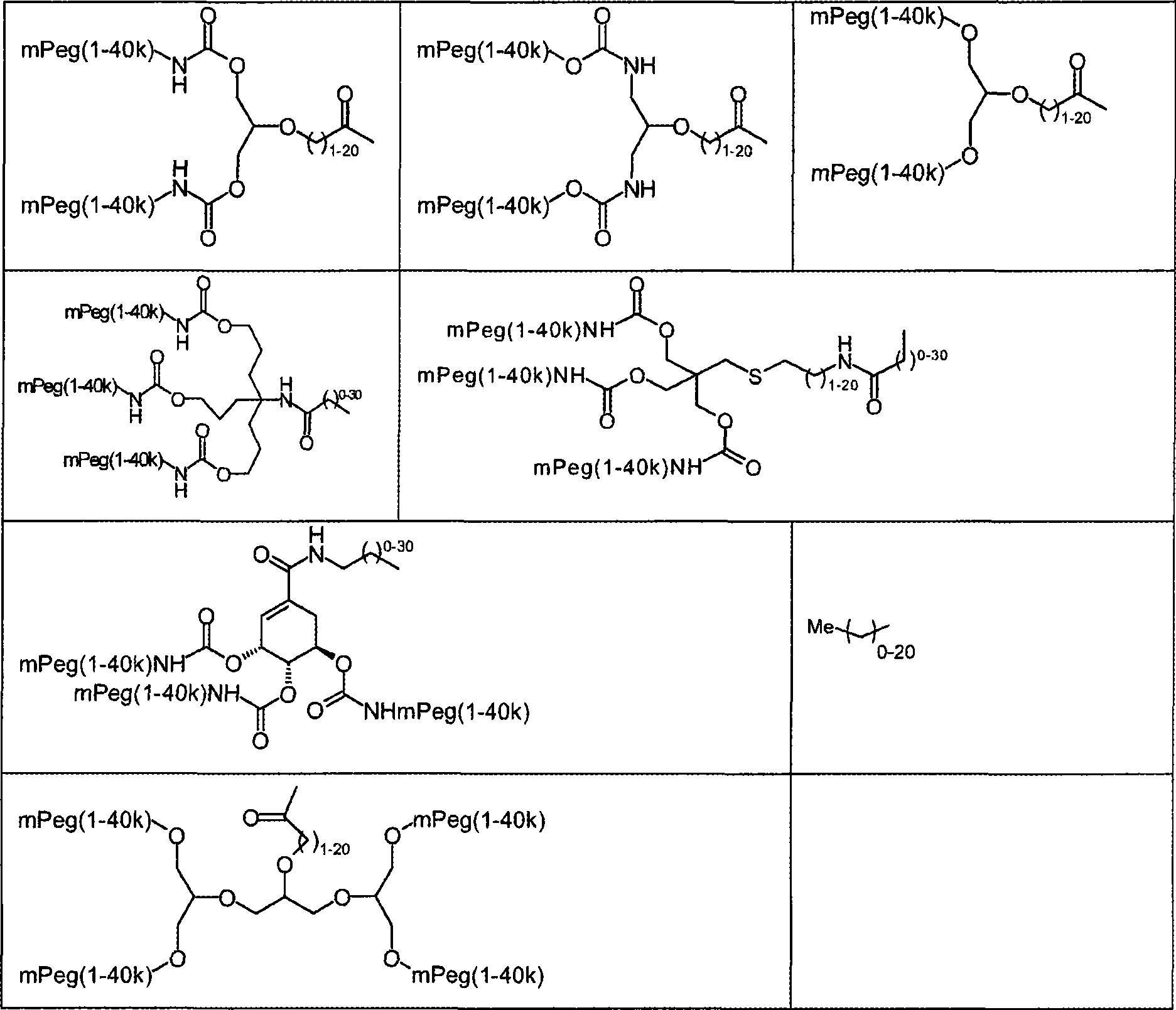
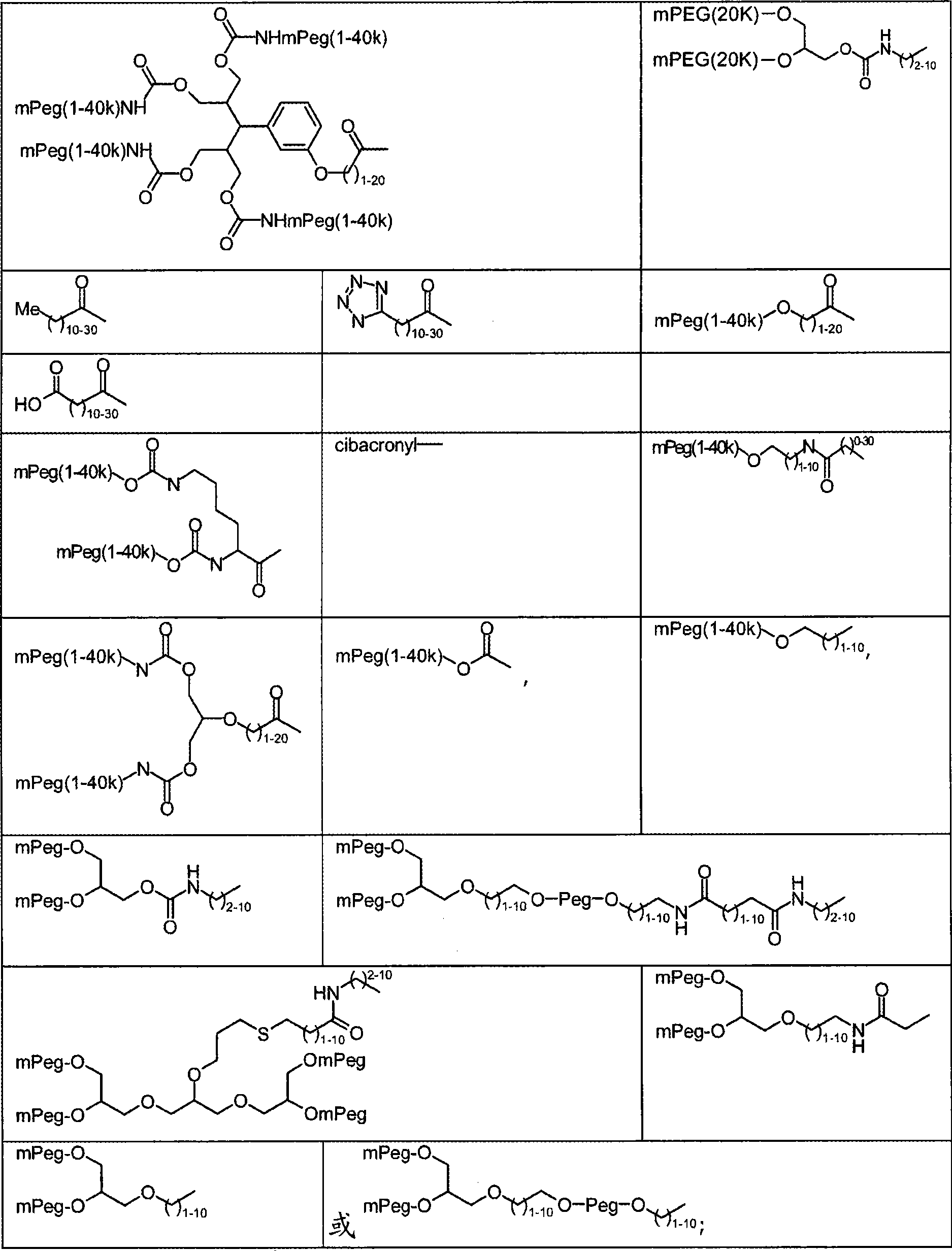
Modified proteins
A glycoprotein modification technology, applied in the field of modified glycoproteins, can solve problems such as accelerated clearance, hindering therapeutic effect, antibody interference, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0248] 10K PEG-ONH 2 : 10K-SMB-PEG (1,00 g; 0.1 mmol; Nektar Inc) was dissolved in DCM (10 ml). 4-(N-tert-butoxycarbonylaminooxy)butylamine (0.20 g, 1 mmol, prepared according to WO 2005014049 A2) was added, and the mixture was stirred at room temperature for 16 h. Diethyl ether (90ml) was added and the white precipitate was filtered off. The steps of dissolving the precipitate with DCM (10 ml) and adding diethyl ether (90 ml) were repeated. The precipitated material was then reconstituted with DCM (6 ml) and Amberlyst 15 ion exchange resin (2,0 g; washed first with DCM and 10% EtOH in DCM) was added. The mixture was stirred at room temperature for 30 minutes, then the resin was filtered off and washed well with DCM. The combined DCM solution was concentrated to a small volume with a rotary evaporator, and then diethyl ether (90ml) was added to precipitate the product. The product was dissolved in DCM (6ml) and TFA (6ml) was added. The mixture was stirred at room temperat...
Embodiment 2
[0251] Single 10K-PEG-FVIIa (0128-0000-1018-1A):
[0252] Dissolve in 10ml 25mM Gly-Gly, 50mM NaCl, 25mM CaCl 2 , Factor VIIa (14mg, 0.28umol) of pH 6.0 (GlyGly buffer) was added to 100ul CaCl 2 20 mM NaIO in free GlyGly buffer 4 solution and 1000ul of 10K-PEG-ONH in GlyGly buffer 2 Solution (Example 1, 42 mg; 4.2 umol; 15 equivalents of Factor Vila): The mixture was placed in an ice bath for 2 h with occasional shaking. Then add 500ul MeONH in GlyGly buffer (1M NaOH to adjust pH to 6.0) in the reaction mixture 2· HCl solution (17 mg; 0, 21 mmol). The mixture was placed in an ice bath for another 10 min. Then 100 mM cold EDTA solution (4, 5 ml) was added to the reaction mixture to maintain the pH below 9.0. Then adjust the pH to 8.0 with 100ul 1N HCl solution.
[0253] Ion Exchange Chromatography:
[0254] Removal of excess PEG-ONH by ion exchange chromatography 2 . The cooled reaction mixture was loaded onto a 5 ml HiTrap Q ion exchange column (Amersham Bioscience) ...
Embodiment 3
[0259] Preparation of highly substituted (HS) and low substituted (LS) 10K-PEG-FVIIa
[0260] Dissolve in 10ml 25mM Gly-Gly, 50mM NaCl, 25mM CaCl 2 100ul CaCl was added to factor VIIa (14mg, 0.28umol) of pH 6.0 (GlyGly buffer) 2 20 mM NaIO in free GlyGly buffer 4 solution and 1000ul of 10K-PEG-ONH in GlyGly buffer 2 Solution (Example 1, 42 mg; 4.2 umol; 15 equivalents to Factor Vila): The mixture was placed at 4° C. for 24 h with occasional shaking. Then add MeONH in 500ul GlyGly buffer (adjust pH to 6.0 with 1M NaOH) to the reaction mixture 2· HCl solution (17 mg; 0, 21 mmol). The mixture was placed in an ice bath for 10 min. A cold 100 mM second generation EDTA solution (4, 5 ml) was then added to the reaction mixture to maintain the pH below 9.0. Then adjust the pH to 8.0 with 60ul 1N HCl solution.
[0261] Ion Exchange Chromatography:
[0262] Removal of excess PEG-ONH by ion exchange chromatography 2 . The cooled reaction mixture was loaded onto a 5 ml HiTrap Q ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Protein concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com