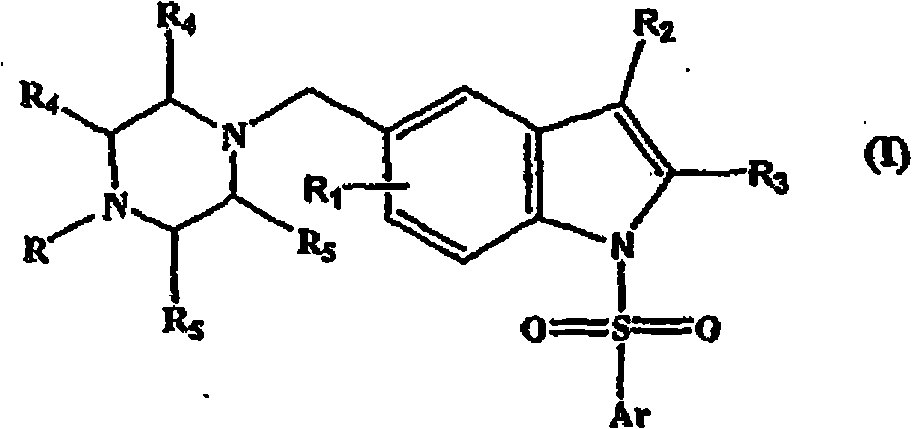
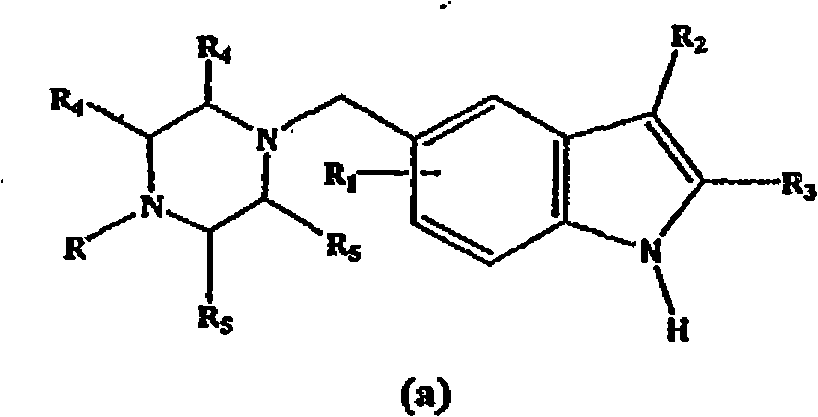
5-(heterocyclyl)alkyl-N-(arylsulfonyl)indole compounds and their use as 5-HT6 ligands
A technology of compound, alkyl, applied in the field of 5-alkyl-N-indole compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Example 1: Preparation of 5-(4-methylpiperazin-1-ylmethyl)-1H-indole
[0141] Step (i): Preparation of (3-methyl-4-nitrophenyl)-(4-methylpiperazin-1-yl)methanone
[0142] 3-Methyl-4-nitrobenzoic acid (5.525 mmol, 1.0 g) was placed in a 25 mL two necked round bottom flask connected to a condenser (with protective tube). Thionyl chloride (6.07 mmol, 0.735 g) and 1,2-dichloroethane (5 mL) were added thereto, and the resulting solution was refluxed for 3 hours. The reaction mixture was added to another 100 mL flask containing a solution of N-methylpiperazine (16.57 mmol, 1.66 g) in 10 mL of 1,2-dichloroethane, where the temperature was maintained below 5°C. The reaction mixture was then stirred at 25°C for 0.5 hours. After the reaction was complete, the reaction mixture was poured into 50 mL of water. The 1,2-dichloroethane layer was separated, washed with water (2×10 mL), brine (10 mL), and dried over anhydrous sodium sulfate. The volatiles were removed under reduced p...
Embodiment 2
[0149] Example 2: Preparation of 1-benzenesulfonyl-5-(4-methylpiperazin-1-ylmethyl)-1H-indole dihydrochloride
[0150] 5-(4-Methylpiperazin-1-ylmethyl)-1H-indole (0.8733 mmol, 0.2 g) (obtained in Example 1) was dissolved in 2 mL of N,N-dimethylformamide. The above solution was then added slowly to a 25 mL flask containing a suspension of sodium hydride (1.31 mmol, 31.4 mg) in 1 mL DMF under nitrogen atmosphere while maintaining the temperature below 10 °C. The reaction mixture was then stirred at 25°C for 1 hour. Benzenesulfonyl chloride (1.31 mmol, 0.2312 g) was slowly added to the well stirred solution while maintaining the temperature below 10°C. The reaction mixture was stirred for an additional 2 hours. After the reaction was completed, the reaction mixture was poured onto 20 g of ice-water mixture with stirring, and the resulting mixture was extracted with ethyl acetate (2×20 mL). The combined ethyl acetate extracts were then washed with water (20 mL), brine (20 mL), ...
Embodiment 3
[0155] The following compounds (2-34) were prepared using the procedure described in Example 2 with some non-critical modifications.
[0156] 2.
[0157] 5.
[0158] 10.
[0159] 14.
[0160] -(4-Methylpiperazin-1-ylmethyl)
[0161] -1H-Indole dihydrochloride
[0162] (2H, m).
[0163] Acyl]-5-(piperazin-1-ylmethyl)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com