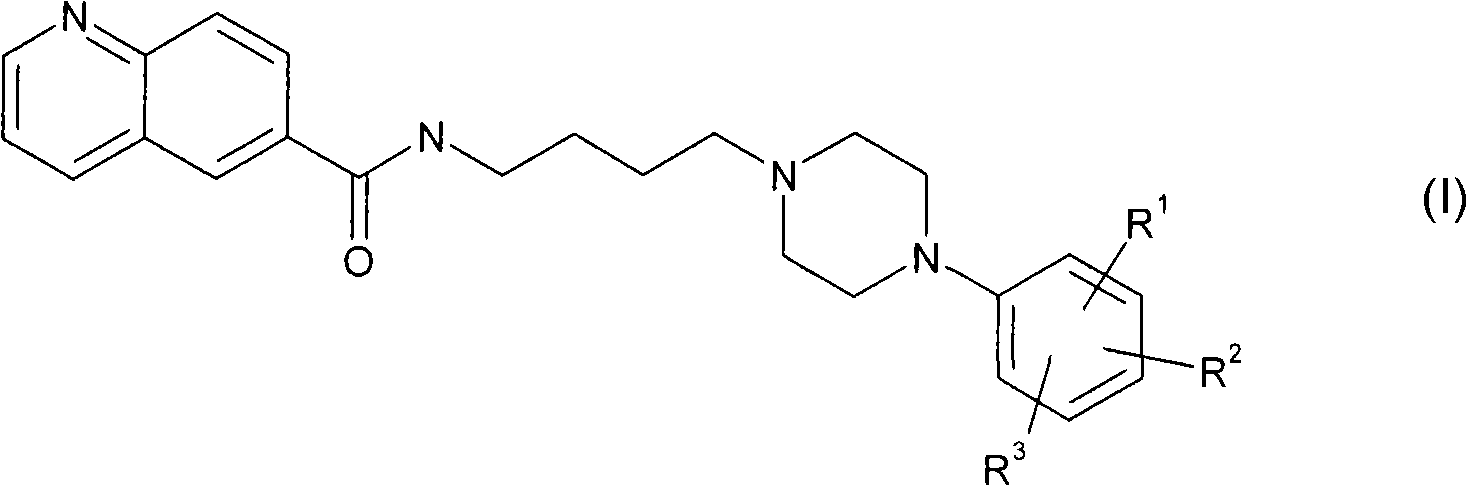
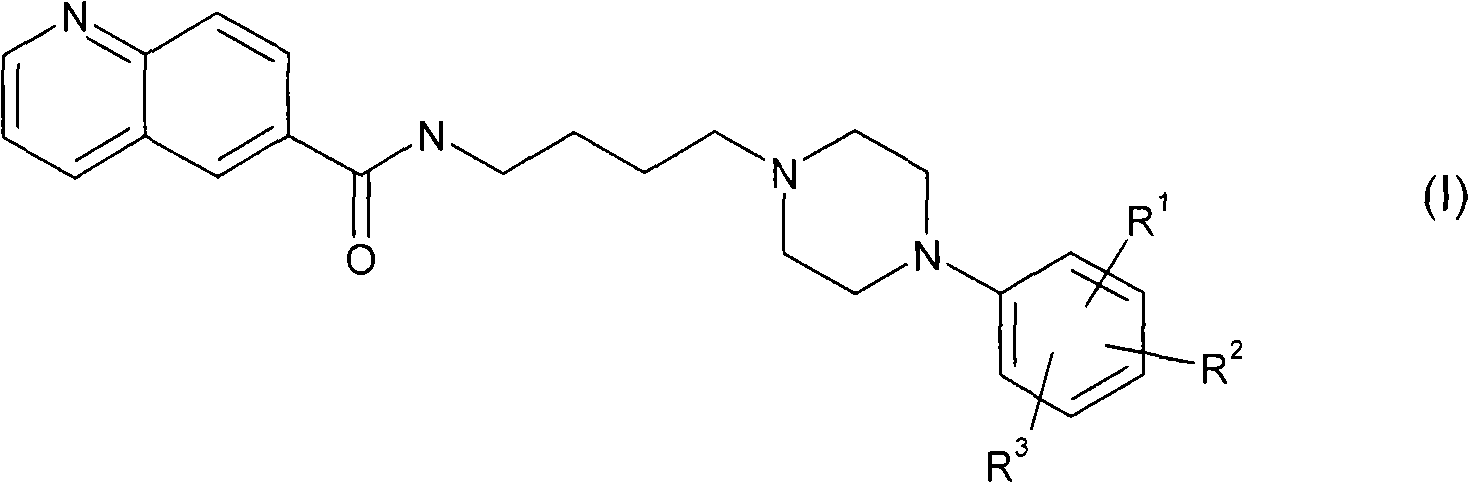
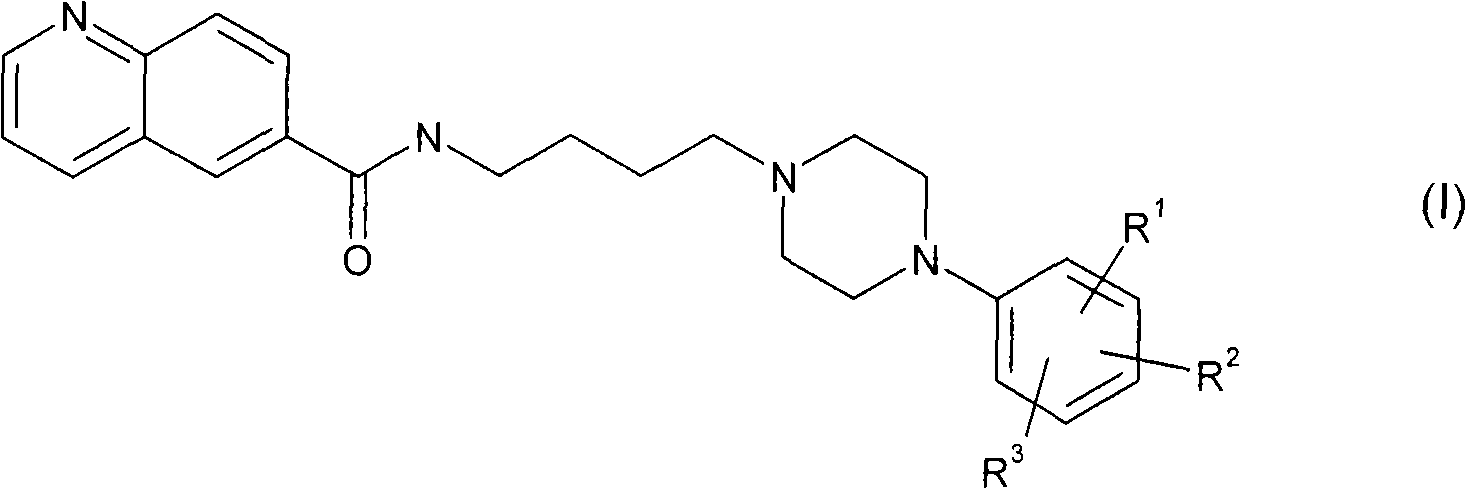
Aryl piperazine derivatives useful for the treatment of neuropsychiatry disorders
A technology of arylpiperazine and derivatives, which is applied in the field of arylpiperazine derivatives, and can solve problems such as no reports of arylpiperazine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Preparation Example
[0084] In order to get 5 1 and 5 2 The synthetic strategy followed is reported in Scheme 1 below.
[0085] Commercially available 6-methylquinoline was oxidized to the corresponding quinoline-6-carboxylic acid (2) in acidic medium by using chromium trioxide. The acid 2 was converted to the hydroxyamide 3 by means of a coupling reaction with 4-aminobutanol in the presence of 1-hydroxybenzotriazole (HOBt) and 1,3-dicyclohexylcarbodiimide (DCC). . The latter, following bromination of the hydroxyl group, leads to the bromo-derivative 4, which is treated with the appropriate arylpiperazine in the presence of a base to give the desired product (5 1,2 ).
[0086] plan 1
[0087]
[0088] a) CrO 3 、H 2 O / H 2 SO 4 Reflux; b) 4-amino-1-butanol, EDC, HOBt, TEA, CH 2 Cl 2 dry, rt; c) CBr 4 、PPh 3 、CH 3 CN, rt; d) for 5 1 1-phenylpiperazine or for 5 2 8, TEA, CH 3 CN, reflow.
[0089] Synthesis of 5 was obtained from Boc-piperazine and 1-b...
Embodiment 2
[0122] biological activity
[0123] In vitro binding studies
[0124] The affinity of Compound 5 of the present invention for dopamine and serotonin receptor subtypes was determined by a standard receptor binding assay performed by MDS Pharma Services using the specific assay conditions described below.
[0125] From these assays, compound 5 of the present invention was found to be selective for dopamine D3 with a Ki in the subnanomolar range.
[0126] Dopamine D3 (MDS Catalog No.219800)
[0127] Human Recombinant CHO Cells
[0128] Ligand = 0.7nM [ 3 H]-Spiperone
[0129] Nonspecific = 25 μM S(-)-sulpiride
[0130] 5-hydroxytryptamine 5-HT 1A (MDS Catalog No.271110)
[0131] Human recombinant (CHO cells)
[0132] Ligand = 1.5nM [ 3 H]8-OH-DPAT
[0133] Nonspecific = 10 μM ergobenzyl ester
[0134] 5-hydroxytryptamine 5-HA 2A (MDS Catalog No.271650)
[0135] Human recombinant (CHO cells)
[0136] Ligand = 0.5nM [ 3 H]ketanserin
[0137] Nonspecifi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com