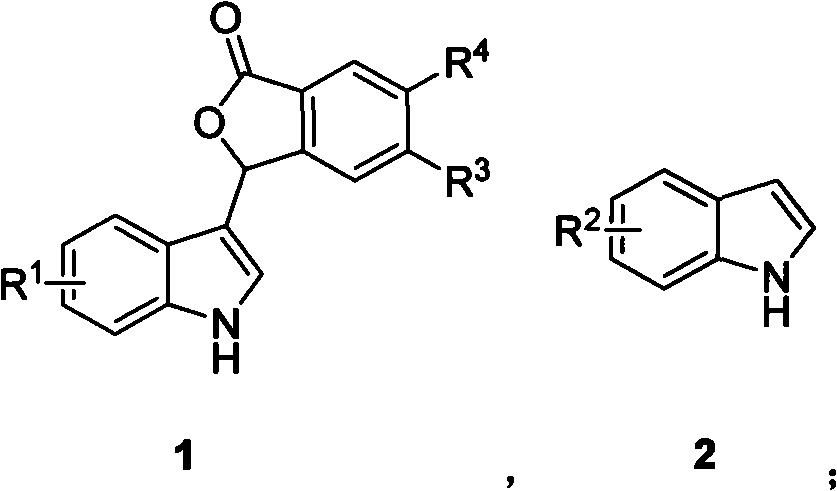
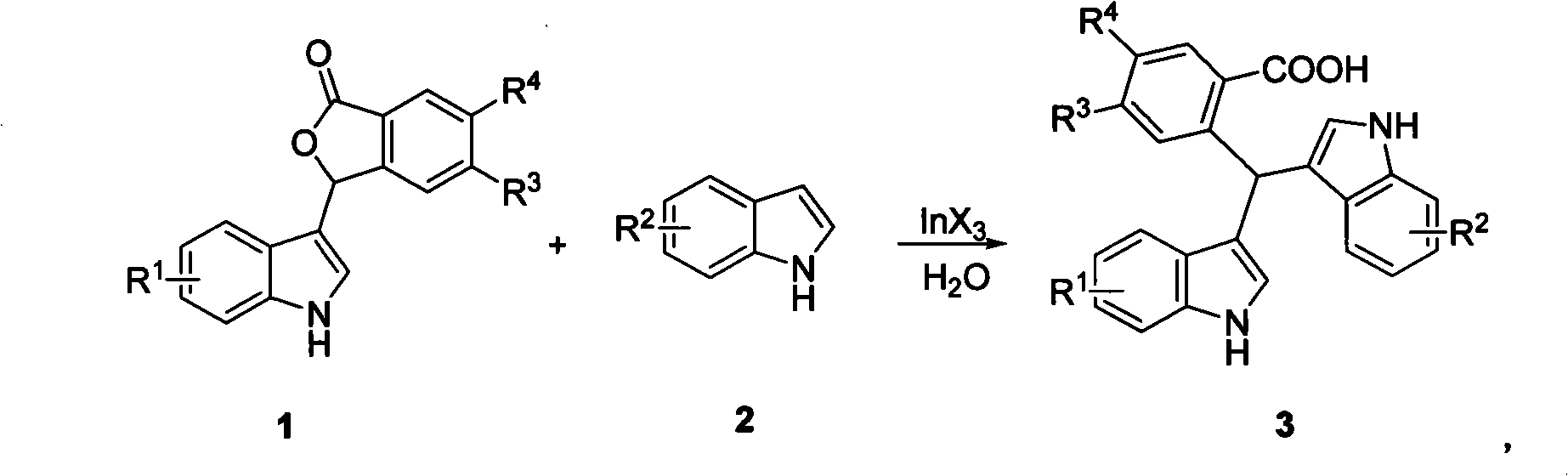
Method for preparing unsymmetrical bis(indolyl)methane compound
A technology for diindolylmethane and compounds is applied in the field of preparing asymmetric diindolylmethane compounds, and achieves the effects of simple reaction system, high atom economy, and good anti-senile dementia effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Synthesis of 3a
[0018] Add 0.5 to 500 mL of water to a dry 1 to 1000 milliliter (ml) reaction flask, and add 0.01 to 10 mmol of InX 3 , 0.2-200mmol substrate 1a and 0.3-300mmol substrate 2a, stir and react at a certain temperature for 5 hours, extract the aqueous phase with ethyl acetate, dry the organic phase with anhydrous sodium sulfate, concentrate, and purify by fast silica gel column chromatography to obtain product 3a .
[0019] White solid 1 H NMR (400MHz, Acetone-d 6 ): δ9.90(s, 1H), 9.79(s, 1H), 7.91(d, J=8.4Hz, 1H), 7.32-7.40(m, 4H), 7.22-7.28(m, 2H), 7.19( s, 1H), 7.16 (s, 1H), 7.05 (t, J=7.2Hz, 7.2Hz, 1H), 6.89 (t, J=7.2Hz, 7.2Hz, 2H), 6.67 (s, 1H), 6.62 (s, 1H), 2.25 (s, 3H); 13 C NMR(125MHz, Acetone-d 6 ): δ169.8, 146.7, 140.0, 136.5, 136.3, 131.9, 131.6, 131.0, 130.9, 128.5, 128.2, 128.0, 126.6, 125.0, 124.8, 124.7, 123.8, 122.1, 120.4, 120.0, 119.4, 112.1, 111.8, 35.9, 21.7; EI-HRMS m / z calculated value C 25 H 20 N 2 O 2 : 380.1525[M] + ; Measured value...
Embodiment 2
[0021] Synthesis of 3b
[0022] The operation is the same as in Example 1.
[0023] White solid 1 H NMR (400MHz, Acetone-d 6 ): δ 11.23 (s, 1H), 9.93 (s, 1H), 9.79 (s, 1H), 7.87-7.91 (m, 1H), 7.42-7.53 (m, 1H), 7.35-7.38 (m, 2H) ), 7.19-7.29 (m, 3H), 7.11 (s, 1H), 7.06 (t, J=8.4 Hz, 7.2 Hz, 1H), 6.85-6.91 (m, 2H), 6.69-6.74 (m, 3H) , 3.60(s, 3H); 13 C NMR(100MHz, Acetone-d 6 ): δ169.2, 153.6, 145.8, 137.3, 132.4, 131.1, 130.8, 130.1, 129.9, 127.8, 127.4, 125.8, 124.7, 124.0, 121.3, 119.7, 118.9, 118.8, 118.7, 118.5, 111.9, 111.3, 101.6, 54.3, 35.1
Embodiment 3
[0025] Synthesis of 3c
[0026] The operation is the same as in Example 1.
[0027] White solid 1 H NMR (400MHz, Acetone-d 6 ): δ11.22(s, 1H), 9.93(s, 1H), 9.81(s, 1H), 7.90(d, J=7.2Hz, 1H), 7.36-7.43(m, 6H), 7.24-7.31( m, 6H), 7.11 (s, 1H), 7.01-7.05 (m, 2H), 6.89 (t, J = 8.0 Hz, 7.2 Hz, 1H), 6.80 (d, J = 8.8 Hz, 1H), 6.72 ( s, 1H), 4.91 (s, 2H); 13 C NMR(100MHz, Acetone-d 6 ): δ169.5, 152.7, 145.8, 138.2, 137.3, 132.5, 131.1, 130.7, 130.1, 128.3, 127.7, 127.5, 127.4, 127.3, 125.8, 124.7, 124.0, 121.3, 119.6, 119.5, 118.9, 118.8, 118.5, 112.0, 111.9, 111.3, 103.2, 70.2, 35.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com