Compounds and uses thereof
A technology for compounds and medicinal salts, applied in the field of compounds and their uses, can solve problems such as poor absorption, chemical instability, and inability to absorb drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
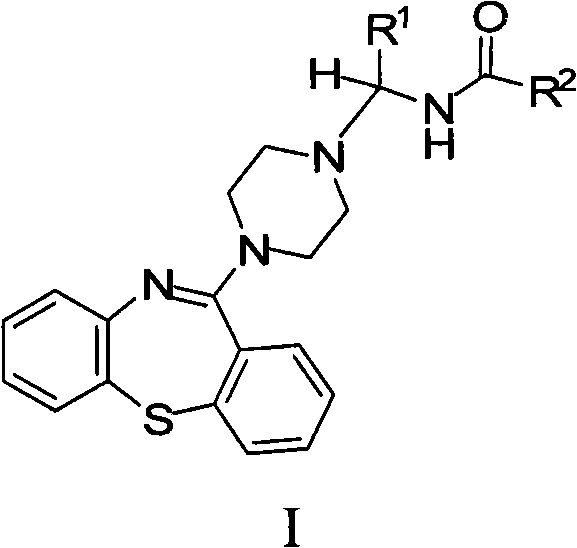
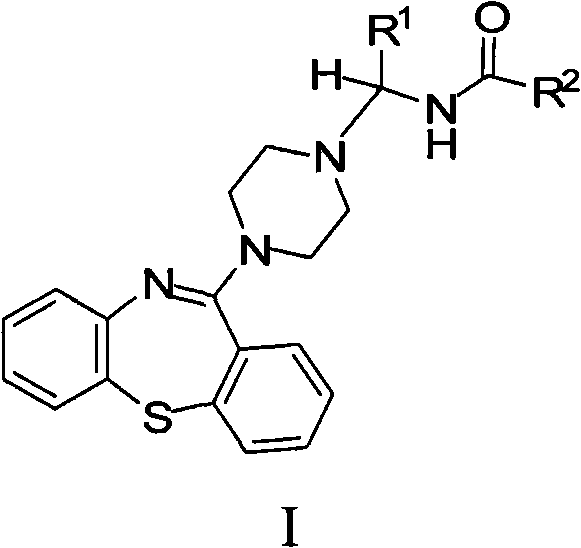
[0182] Example 1: N-((4-(Dibenzo[b,f][1,4]thiazepine -11-yl)-piperazin-1-yl)methyl)-acetamide
[0183]
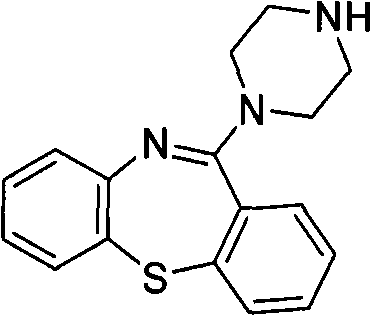
[0184] 11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine ("PDBTZ", 1 mmol), a mixture of 37% aqueous formaldehyde (1 mmol) and acetamide (1 mmol) in ethanol (5 ml) was refluxed for 4 hours. The reaction mixture was evaporated, the residue resuspended in water and the resulting aqueous material extracted with chloroform. The organic portion was washed (water, brine), dried (sodium sulfate) and evaporated to dryness. The crude product was purified by flash chromatography to afford the title compound.
Embodiment 2
[0185] Example 2: N-[1-(4-(dibenzo[b,f][1,4]thiazepine -11-yl)-piperazin-1-yl)-ethyl]-2,2,2-trifluoro-acetamide
[0186]
[0187] A mixture of PDBTZ (1 mmol), acetaldehyde (1 mmol) and 2,2,2-trifluoro-acetamide (1 mmol) in ethanol (5 ml) was refluxed for 4 hours. The reaction mixture was evaporated, the residue resuspended in water and the resulting aqueous material extracted with chloroform. The organic portion was washed (water, brine), dried (sodium sulfate) and evaporated to dryness. The crude product was purified by flash chromatography to afford the title compound.
Embodiment 3
[0188] Embodiment 3: mouse animal assay
[0189] Dopamine antagonism of the compounds and compositions of the invention can be evaluated in rodent models. The methods and procedures used can be found in J. Med. Chem., 2001, 44, 372-389, which is hereby incorporated by reference in its entirety. Brain Serotonin 5-HT 2 Results of receptor binding affinity and for dopamine D 1 and D 2 Results of receptor binding affinity. It is hypothesized that a combination of serotonin receptor antagonism and dopamine receptor antagonism, where 5-HT 2 Receptor affinity relative to D 2 Receptor affinity was high, indicating that the compound is a potent atypical antipsychotic. J. Goldstein, "Quetiapine Fumarate (Seroquel): a new atypical antipsychotic," 35(2) Drugs of Today 1993-210 (1999), which is hereby incorporated by reference in its entirety.
[0190] Additionally, compounds and compositions of the invention can be tested for their activity according to the standard apomorphine cli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com