Calcium acetate tablet and preparation method thereof
A technology of calcium acetate and magnesium stearate, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, anhydride/acid/halide active ingredients, etc. The problem of large dosage, etc., achieves the effect of safe and effective treatment of hyperphosphatemia, shortened disintegration time and convenient administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
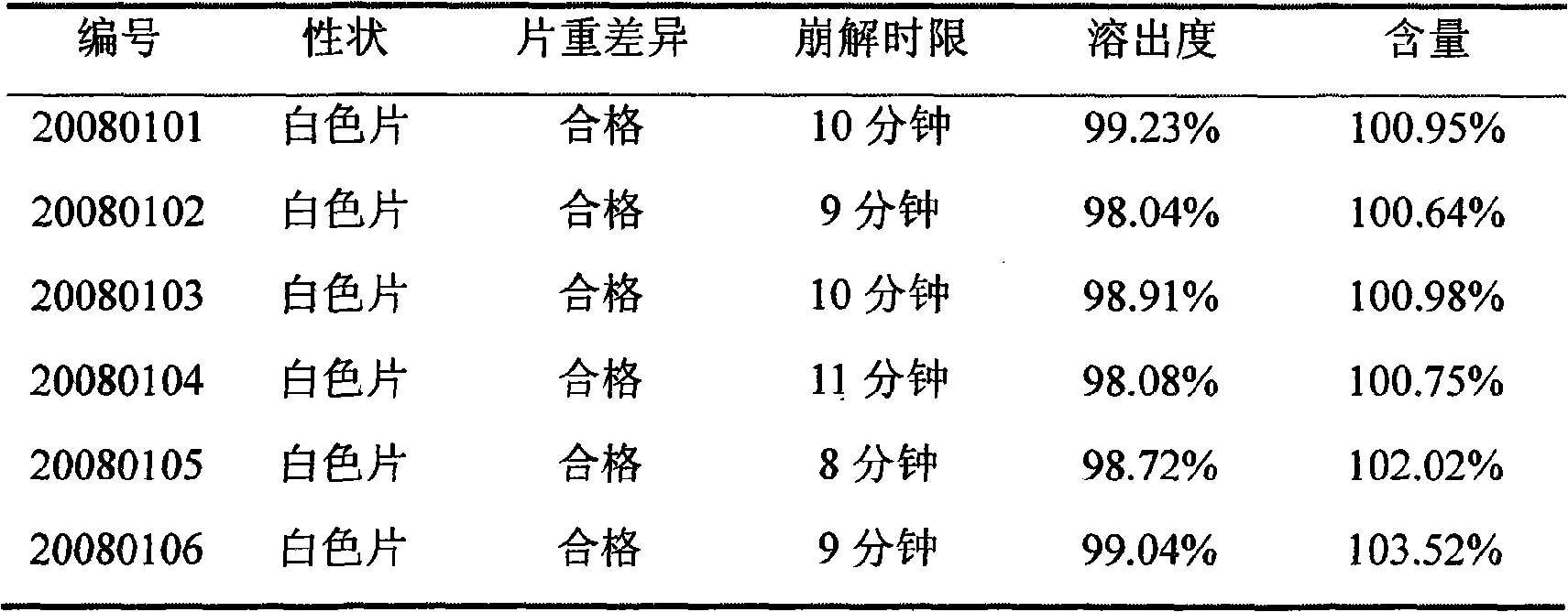
Examples
Embodiment 1
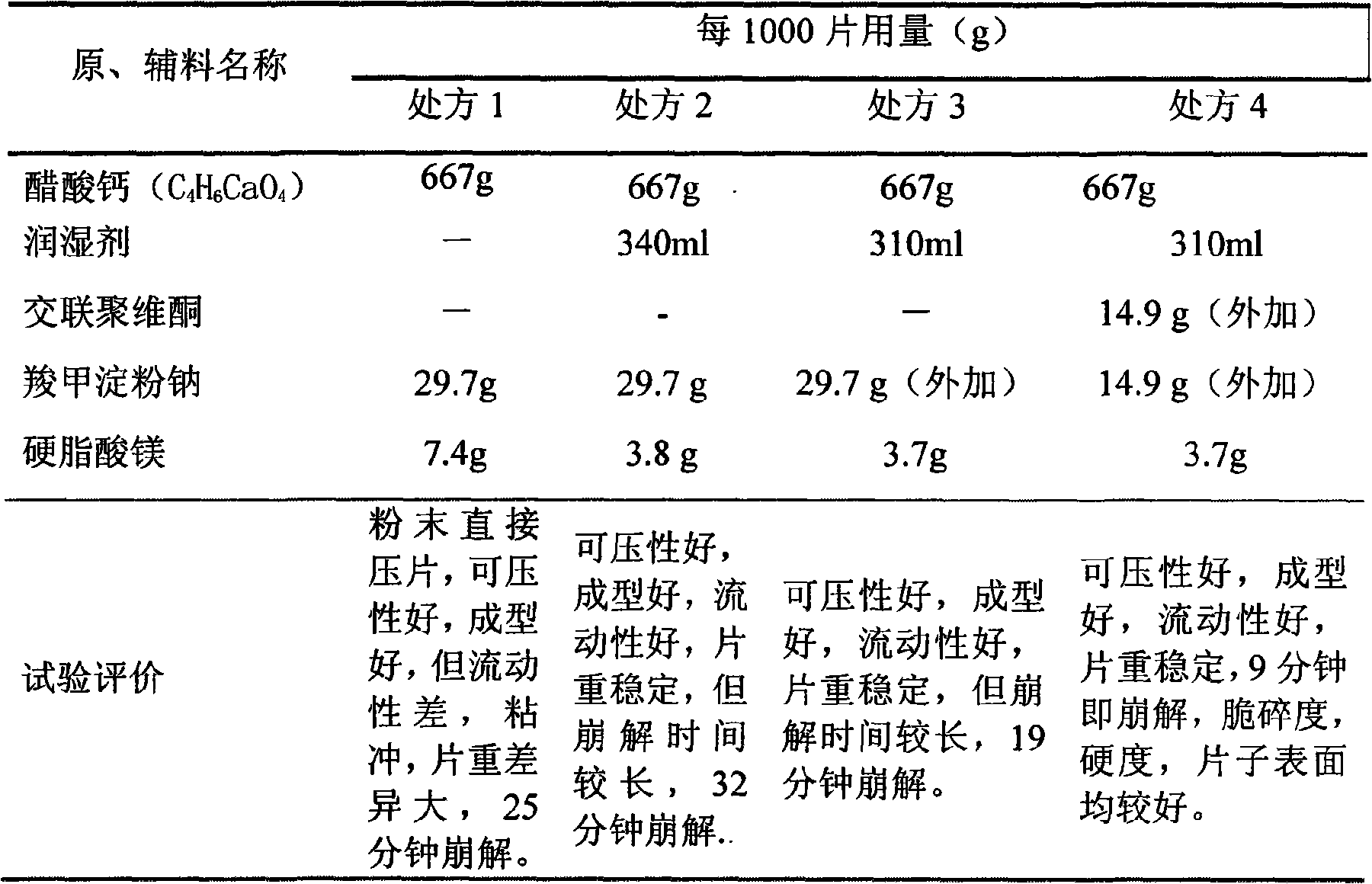
[0028] Calcium acetate (C 4 h 6 CaO 4 ) 667g (91.36%)
[0029] Sodium Starch Carboxymethyl 35g (4.75%)
[0030] Crospovidone 25g (3.39%)
[0031] Magnesium Stearate 10g (1.36%)
[0032]
[0033] Makes 1000 pieces
[0034] Preparation method:
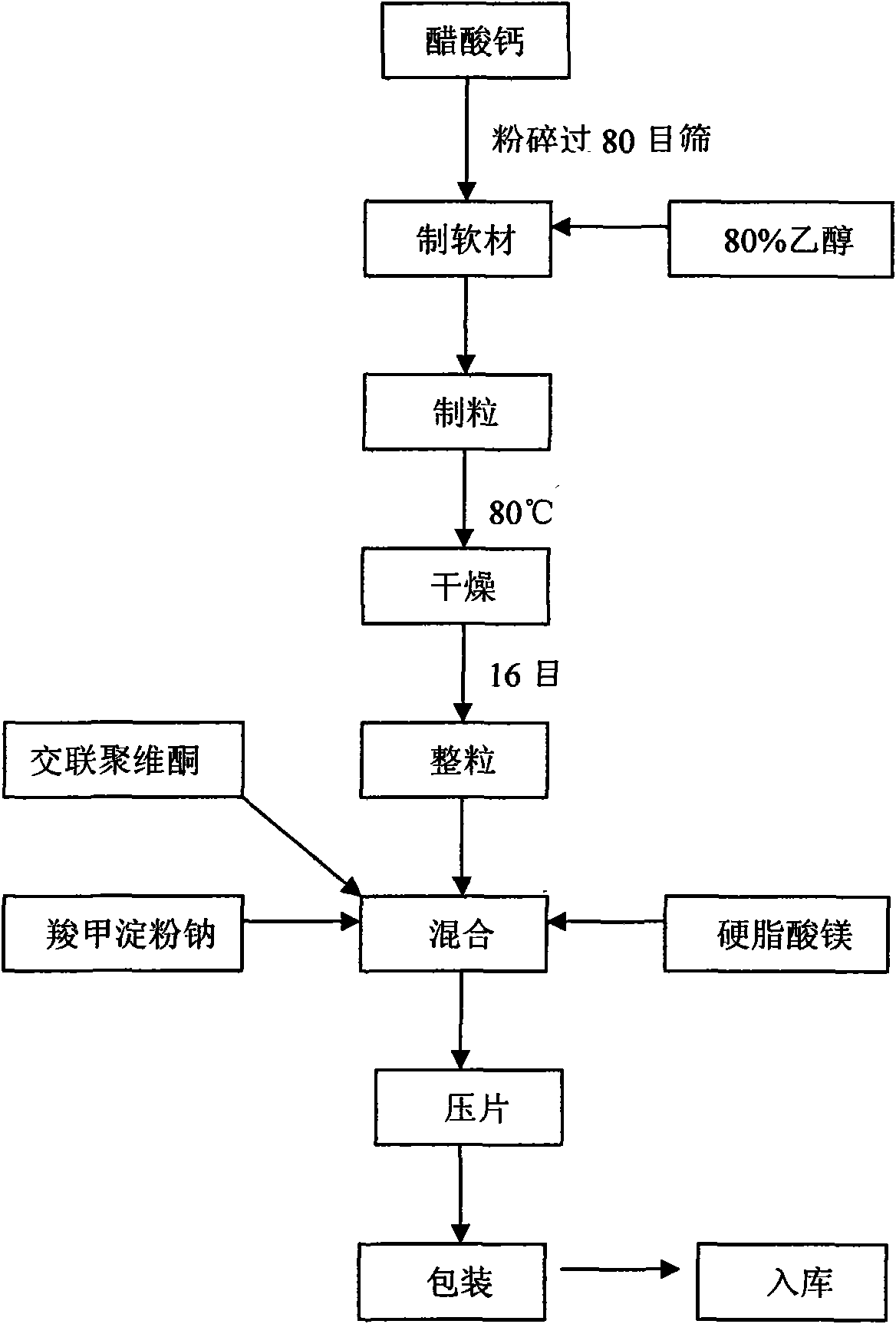
[0035] a. Calcium acetate is pulverized through a 60-mesh sieve for subsequent use;
[0036] b. Prepare 50% ethanol for subsequent use;
[0037] c. Take the calcium acetate of recipe quantity and put in the fast mixing granulator, add 50% ethanol in right amount, make soft material, cross 10 mesh sieves and granulate;
[0038] d. Set the drying temperature to 50°C, and dry the wet granules in a hot air circulation oven or common drying equipment until the moisture content is lower than 5%;
[0039] e. Sieve the dry granules through 16 sieves, add sodium starch glycolate, crospovidone and magnesium stearate, and mix in a mixer for 7 minutes;
[0040] f. Measure the main drug co...
Embodiment 2
[0043] Calcium acetate (C 4 h 6 CaO 4 ) 667g (95.68%)
[0044] Sodium starch glycolate 14.9g (1.92%)
[0045] Crospovidone 14.9g (1.92%)
[0046] Magnesium Stearate 3.7g (0.48%)
[0047]
[0048] Makes 1000 pieces
[0049] Preparation method:
[0050] a. Calcium acetate is pulverized and sieved through 80 sieves for subsequent use;
[0051] b. Prepare 80% ethanol for subsequent use;
[0052] c. take the calcium acetate of recipe quantity and put in the fast mixing granulator, add 80% ethanol in right amount, make soft material, cross 16 mesh sieves and granulate;
[0053] d. Set the drying temperature to 75°C, and dry the wet granules in a hot air circulation oven or common drying equipment until the moisture content is lower than 5%;
[0054] e. Pass the dry granules through a 16-mesh sieve for granulation, add sodium starch glycolate, crospovidone and magnesium stearate, and mix in a mixer for 10 minutes;
[005...
Embodiment 3
[0058] Calcium acetate (C 4 h 6 CaO 4 ) 667g (96.95%)
[0059] Sodium carboxymethyl starch 8g (1.16%)
[0060] Crospovidone 12g (1.74%)
[0061] Magnesium Stearate 1g (0.145%)
[0062]
[0063] Makes 1000 pieces
[0064] Preparation method:
[0065] a. Calcium acetate is pulverized through a 100-mesh sieve for subsequent use;
[0066]b. Prepare 90% ethanol for subsequent use;
[0067] c. take the calcium acetate of recipe quantity and put in the fast mixing granulator, add 90% ethanol in right amount, make soft material, cross 20 mesh sieves and granulate;
[0068] d. Set the drying temperature to 90°C, and dry the wet granules in a hot air circulation oven or common drying equipment until the moisture content is lower than 5%;
[0069] e. Sieve the dry granules through a 20-mesh sieve, add sodium starch glycolate, crospovidone and magnesium stearate, and mix in a mixer for 15 minutes;
[0070] f. Measure the...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap