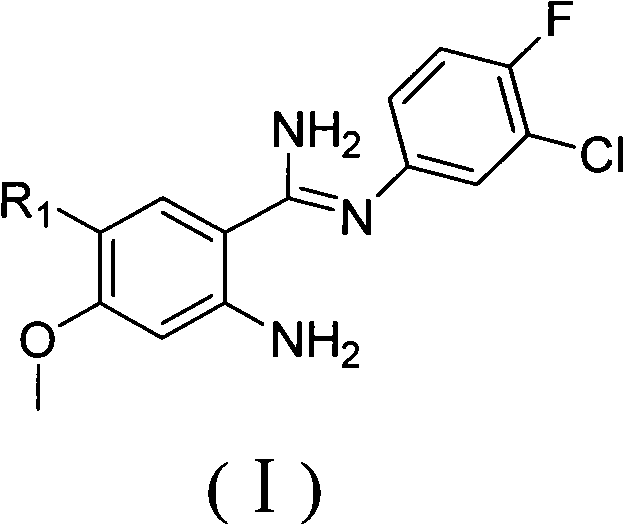
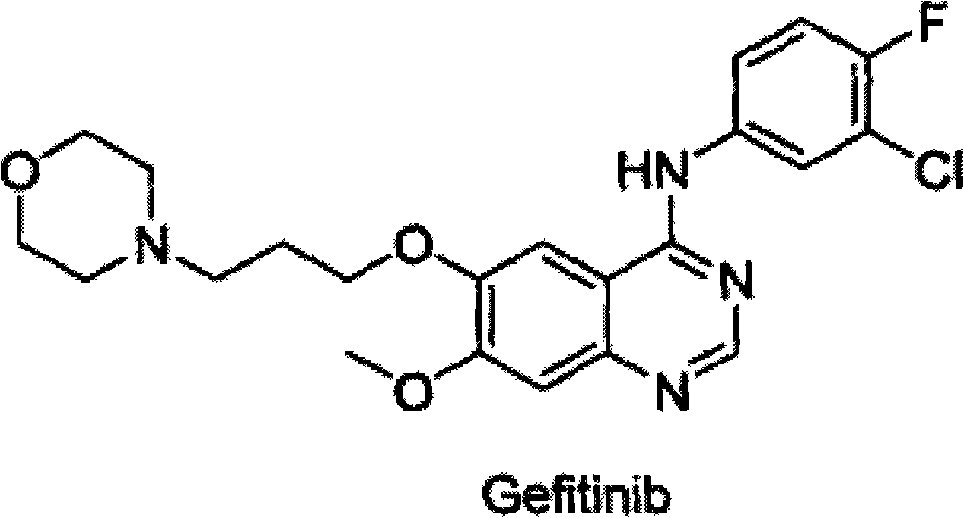
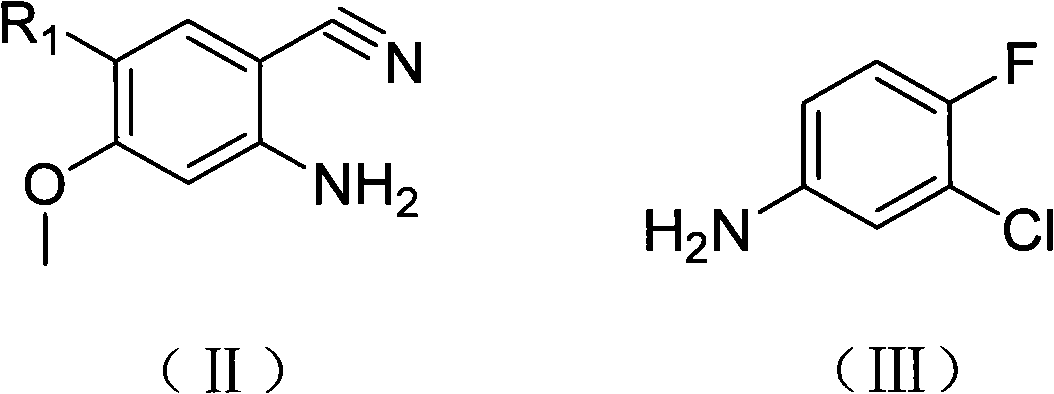Amidine compound capable of preparing gefitinib and preparation method thereof
A technology of gefitinib and compounds, applied in the field of drug preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Preparation of amidine compounds
[0038]
Embodiment 1
[0040] Transfer 0.5 moles (72.8 g) of 3-chloro-4-fluoroaniline and an equivalent molar amount of anthranilocyanide derivatives into a glass reaction vessel, mix well, protect under a nitrogen atmosphere, heat to 50 degrees to start melting, and heat to 80 ~ 100 degrees, the solid basically disappears completely, and a small amount of anhydrous AlCl is added several times under the condition of stirring 3 After the addition, the reaction mixture was heated to 180°C and kept for 30 minutes, then the heating was stopped, and the reactant was down to room temperature, and 200 ml of cold 10% hydrochloric acid solution was added dropwise under the condition of cooling in an ice-water bath, and the reactant was completely dissolved. Mole / liter concentration of sodium hydroxide solution adjust the solution to PH=6, extract 3 times with 100ml chloroform, separate the water phase, alkalinize with 2 moles / liter concentration of sodium hydroxide solution, a off-white solid precipitates, fi...
Embodiment 2
[0042] Transfer 0.5 moles (72.8 g) of 3-chloro-4-fluoroaniline and an equivalent molar amount of anthranilic cyanide derivatives into a glass reaction vessel, mix well, protect under a nitrogen atmosphere, heat until the solid disappears completely, and stir Add small amounts of anhydrous AlCl several times 3 , after the addition, the reaction mixture was heated to 150°C and kept for 50 minutes, then the heating was stopped, the reactant was down to room temperature, and 200ml of cold 10% hydrochloric acid solution was added dropwise under the condition of cooling in an ice-water bath, and the reactant was completely dissolved. Mole / liter concentration of sodium hydroxide solution adjust the solution to PH=6, extract 3 times with 100ml chloroform, separate the water phase, alkalinize with 2 moles / liter concentration of sodium hydroxide solution, a off-white solid precipitates, filter , and the solid recrystallized after drying. The yield is below 50%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap