Skin-lightening agent containing equol compound as active ingredient
A technology of equol and whitening agent, applied in the field of whitening agent, can solve the problems of other functions and applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The glycotransferase used in the preparation of the equol glycoside used in the present invention is not particularly limited as long as it is a glycotransferase that generates equol glycoside when it acts on a solution containing equol and an α-glucosyl sugar compound. . Specifically, for example, α-glucosidase, cyclomaltodextrin glucanotransferase (hereinafter sometimes abbreviated as “CGTase”), α-amylase, etc. can be illustrated. The sources and types of these enzymes are not particularly limited as long as they produce the equol glycoside used in the present invention, and may be enzymes derived from any of animals, plants, molds, bacteria, and yeasts, From the viewpoint of ease of production and safety, it is preferable to use enzymes derived from microorganisms, and from the viewpoint of production efficiency of glycosides, CGTase is particularly preferable. In addition, these glycotransferases do not necessarily need to be purified before use. For example, when ...
Embodiment 1
[0170]
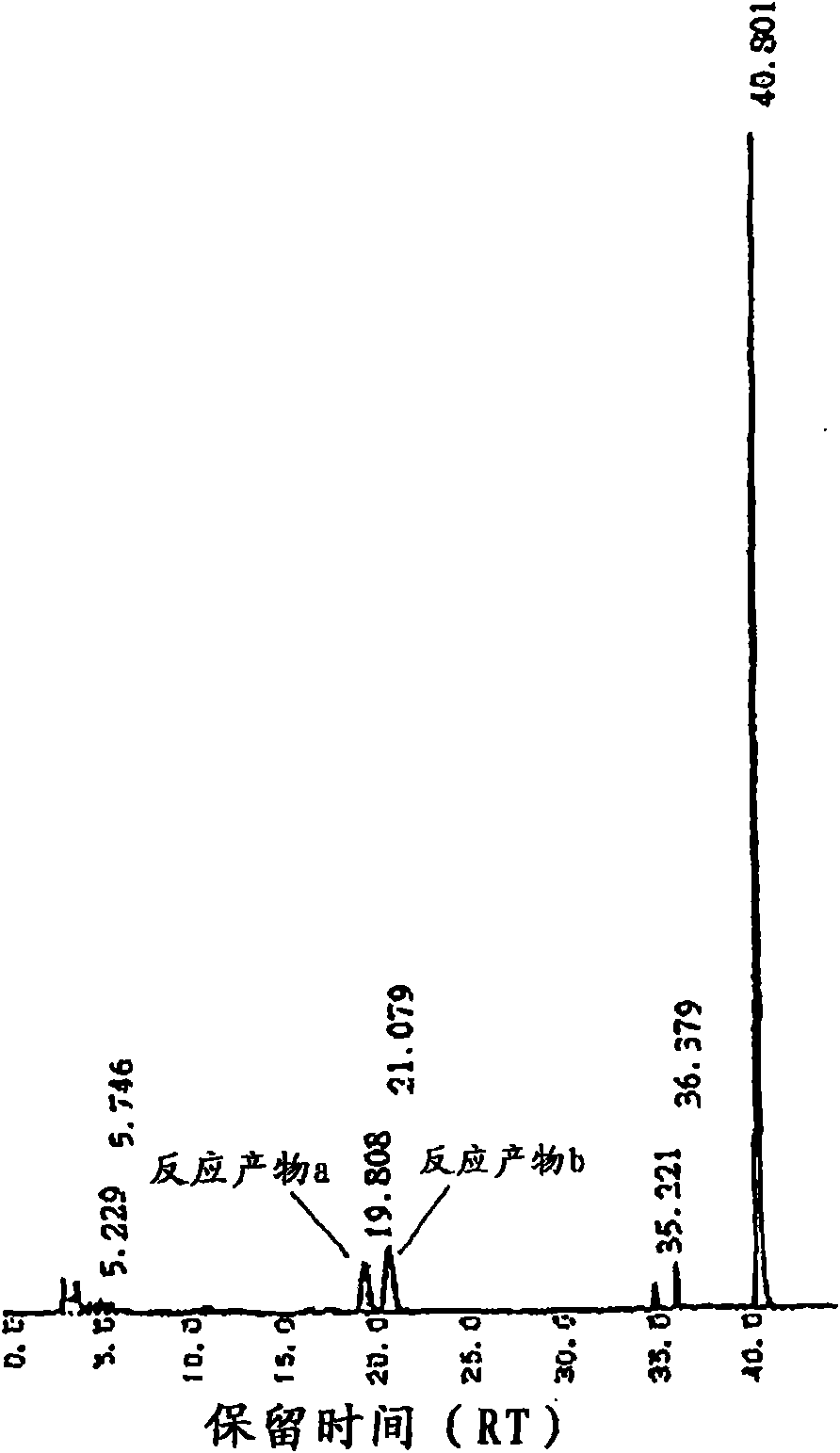
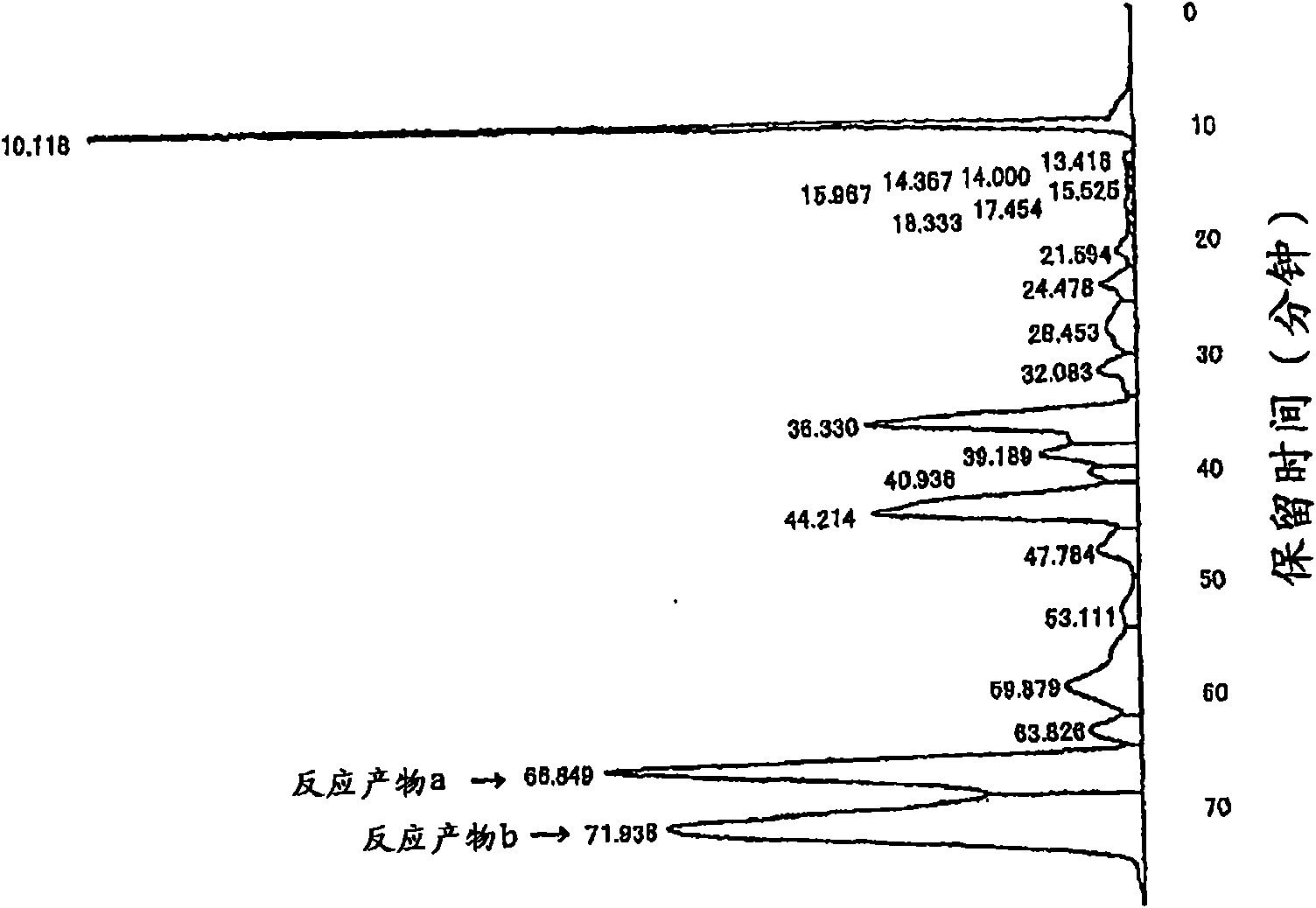
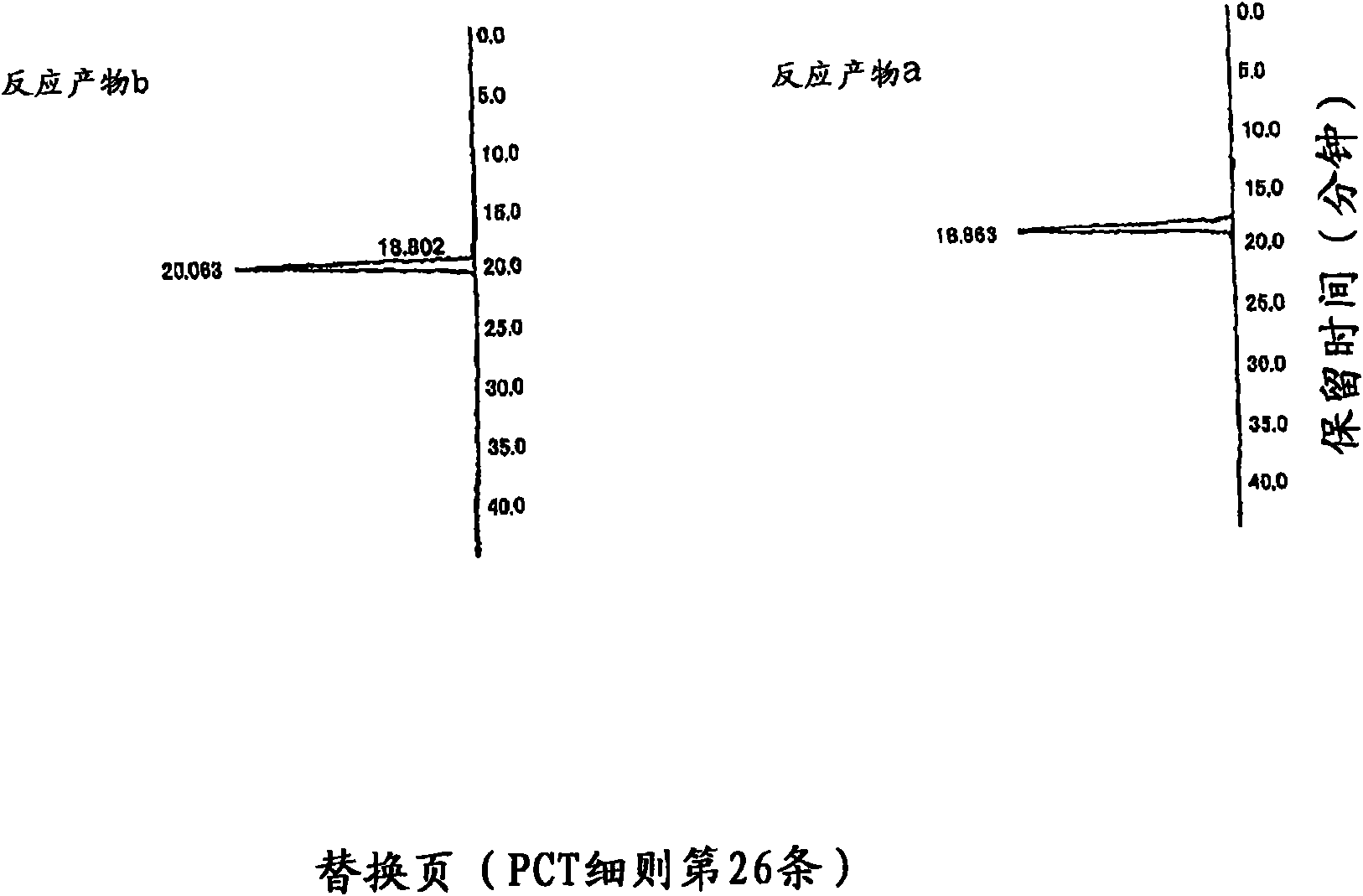
[0171] After dissolving 1 g of reagent grade equol (sold by Nacalai Tesque Co., Ltd., product code "26355-54", a mixture of R-equol and S-equol) into 100 ml, mix it in the bag Containing 100 g of dextrin (sold by Matsutani Chemical Industry Co., Ltd., trade name "Pinedex #1"), 50 mM acetate buffer solution of pH 6.0, and 1000 ml of 2 mM calcium chloride solution, then, add CGTase (manufactured by Hayashibara Biochemical Laboratory Co., Ltd.) was used at 1000 U / g, and cultured at 40° C. for 72 hours. After heating at 100° C. to terminate the reaction, 70 ml of 1 M acetate buffer solution and 10 U / g of glucoamylase (manufactured by Seikagaku Kogyo) were added, and cultured at 40° C. for 18 hours. The resulting reaction solution was supplied to a chromatographic column (3 cmΦ x 90 cm, 640 ml) filled with a porous synthetic adsorbent (sold by Mitsubishi Chemical Corporation, trade name "diaion HP-20 chromatographic column"), and the chromatographic column was washed...
Embodiment 2
[0174]
[0175] Use pharmaceutical grade equol (sold by Funakoshi Co., Ltd., a mixture of S-equol and R-equol), and use the same method as Experiment 1 to separate into S-equol and R-equol . 1 g of the obtained R-equol was dissolved in 100 ml of ethanol, and mixed with 100 g of dextrin (sold by Matsutani Chemical Industry Co., Ltd., trade name "Pinedex #1"), pH 6.0, 50 mM acetate buffer solution, 2 mM Then, 1000 U / g of CGTase (manufactured by Hayashibara Biochemical Laboratory Co., Ltd.) derived from Bacillus stearothermophilus was added to 1000 ml of calcium chloride solution, and cultured at 40° C. for 72 hours. After heating at 100°C to terminate the reaction, 100 ml of 1M acetate buffer solution and 10 U / g of glucoamylase (manufactured by Seikagaku Kogyo) were added, and cultured at 40°C for 18 hours. The resulting reaction solution was supplied to a chromatographic column (3 cmΦ x 90 cm, 640 ml) filled with a porous synthetic adsorbent (sold by Mitsubishi Chemical ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com