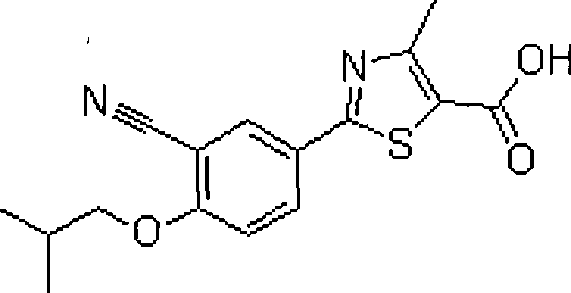
Febuxostat enteric preparation
An enteric-coated preparation, the technology of febuxostat, applied in the field of enteric-coated preparations of febuxostat, can solve the problems of gastrointestinal irritation, gastric ulcer, easily damaged gastric mucosa, etc., achieve fast dissolution and absorption, and high bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
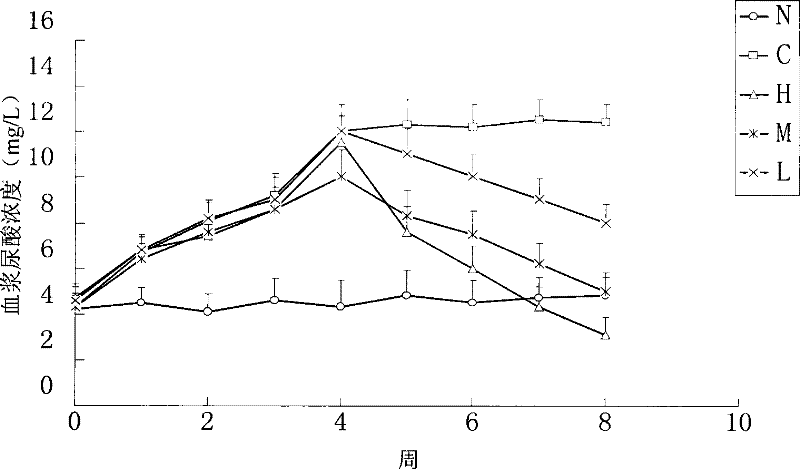
Image
Examples
Embodiment 1
[0021] Embodiment 1 enteric-coated febuxostat tablet
[0022] (1) chip core
[0023] Prescription 1000 tablets
[0024] Febuxostat 80g
[0025] 20g pregelatinized starch
[0026] Hydroxypropyl Cellulose 10g
[0027] Preparation: Take febuxostat, pregelatinized starch, and hydroxypropyl cellulose and pulverize them into an 80-mesh sieve respectively, weigh and mix according to the prescription amount, and use 2% ethanol aqueous solution of hydroxypropyl methyl cellulose (80% ) appropriate amount, the soft material is passed through a 16-mesh sieve, granulated, dried at 50-60°C, and granulated with a 18-mesh sieve. Measure the content of granules, press into tablets, and obtain. Each tablet weighs 120mg and contains 80mg of febuxostat.
[0028] (2) isolation layer
[0029] prescription
[0030] Hypromellose Phthalate 2g
[0031] Tween-80 2g
[0032] Ethanol 80ml
[0033] water 20ml
[0034] Dosing: Take the prescribed amount of hypromellose phthalate and disperse it ...
Embodiment 2
[0043] Example 2 Enteric-coated febuxostat pellets
[0044] (1) Prescription of pellets
[0045] Febuxostat 80g
[0046] Lactose 250g
[0047] Silicified Microcrystalline Cellulose 50g
[0048] Preparation: take three kinds of raw materials and auxiliary materials, and prepare it with 2% polyvinylpyrrolidone aqueous solution, and the diameter is 0.5-0.6mm.
[0049] (2) Package isolation layer
[0050] prescription
[0051] Hydroxypropyl Methyl Cellulose Phthalate 5g
[0052] Tween-80 3g
[0053] Ethanol 80ml
[0054] water 20ml
[0055] Preparation and coating are the same as in Example 1, the rotating speed of the coating pan is adjusted to 50 rpm, the air temperature is controlled at about 40°C, the gap is sprayed with an isolation layer, the weight of the pellets is increased by 2%, and it is taken out and dried at 40°C.
[0056] (3) Enteric coating
[0057] prescription
[0058] Polyvinyl acetate phthalate 12g
[0059] Diethyl phthalate 2g
[0060] Stearic acid ...
Embodiment 3
[0063] Embodiment 3 enteric-coated febuxostat tablet
[0064] (1) chip core
[0065] Prescription 1000 tablets
[0066] Febuxostat 120g
[0067] 40g pregelatinized starch
[0068] Hydroxypropyl Cellulose 20g
[0069] Preparation: Same as Example 1, each tablet weighs 180 mg, containing 120 mg of febuxostat.
[0070] (2) isolation layer
[0071] prescription
[0072] Hypromellose Phthalate 10g
[0073] Tween-80 5g
[0074] Ethanol 80ml
[0075] water 20ml
[0076] Dosing: with embodiment 1
[0077] Preparation: with embodiment 1
[0078] Weight gain 4mg per tablet
[0079] (3) Enteric coating
[0080] prescription
[0081] Cellulose acetate 10g
[0082] Phthalic acid 6g
[0083] Ethanol 80ml
[0084] water 20ml
[0085] The preparation and coating are the same as the isolation layer, each tablet has a weight gain of 18 mg, and then taken out and dried overnight at 40°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com