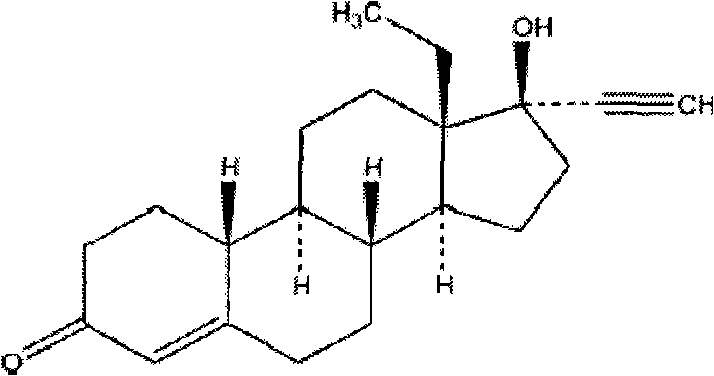
A kind of emergency contraceptive pharmaceutical composition containing levonorgestrel and its preparation method
A technology of levonorgestrel and emergency contraception, which is applied in the directions of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulations, and can solve the problem of failure to reduce the dosage of levonorgestrel and the irrelevance of bioavailability It can not reflect the real dissolution rate, etc., to achieve the effect of low equipment requirements, reduction of individual differences, and the same dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] prescription:
[0053] Levonorgestrel 2.0g
[0054] Organic solvent Trichloromethane 30ml
[0055] Crystallization Inhibitor Povidone K30 20g
[0056] Inert carrier hydroxypropyl cellulose 10g, mannitol 70g, dextrin 20g and microcrystalline cellulose 50g Pharmaceutically acceptable drug carrier sodium carboxymethyl starch 4.8g and magnesium stearate 1.6g
[0057] Preparation:
[0058] (1) adding levonorgestrel to the organic solvent and dissolving to form mixed solution A;
[0059] (2) adding a crystallization inhibitor in A to form a mixed solute B;
[0060] (3) B is slowly added to the inert carrier, granulated (cutting 1500rpm / min, stirring paddle 250rpm / min) to form mixture C;
[0061] (4) Dry C at 75°C for 2 to 3 hours, and the solvent residue should not exceed the limit to obtain mixture D, which is sieved through a 40-mesh sieve to granulate;
[0062] (5) mixture D is mixed with pharmaceutically acceptable medicine, mixes 10min, obtains mixture E;
[0063]...
Embodiment 2
[0065] prescription
[0066] Levonorgestrel 2.0g
[0067] Organic solvent Dichloromethane 30ml
[0068] Crystallization Inhibitor Povidone K30 20g
[0069] Inert carrier methyl cellulose 10g, mannitol 70g, dextrin 20g and microcrystalline cellulose 50g Pharmaceutically acceptable pharmaceutical carrier sodium carboxymethyl starch 4.8g and magnesium stearate 1.6g
[0070] Preparation:
[0071] Steps (1) to (5) are as in Example 1, and step (6) is to press E into tablets with a specification of 1.10 mg (B1) or 1.20 mg (B2) or fill them into capsules B3 and B4 of corresponding specifications. .
Embodiment 3
[0073] prescription
[0074] Levonorgestrel 2.0g
[0075] Organic solvent dimethyl sulfoxide 30ml
[0076] Crystallization Inhibitor Povidone K17 10g
[0077] Inert carrier ethyl cellulose 10g, mannitol 70g, dextrin 20g and microcrystalline cellulose 50g Pharmaceutically acceptable pharmaceutical carrier sodium carboxymethyl starch 4.8g and magnesium stearate 1.6g
[0078] Preparation:
[0079] Steps (1) to (5) are as in Example 1, and step (6) is to press E into tablets with a specification of 1.10 mg (C1) or 1.20 mg (C2) or fill them into capsules C3 and C4 of corresponding specifications. .
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap