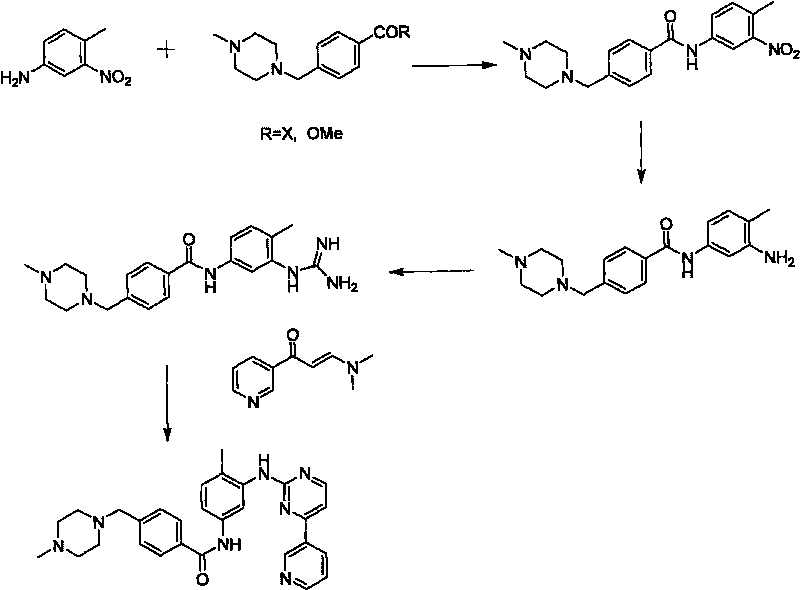
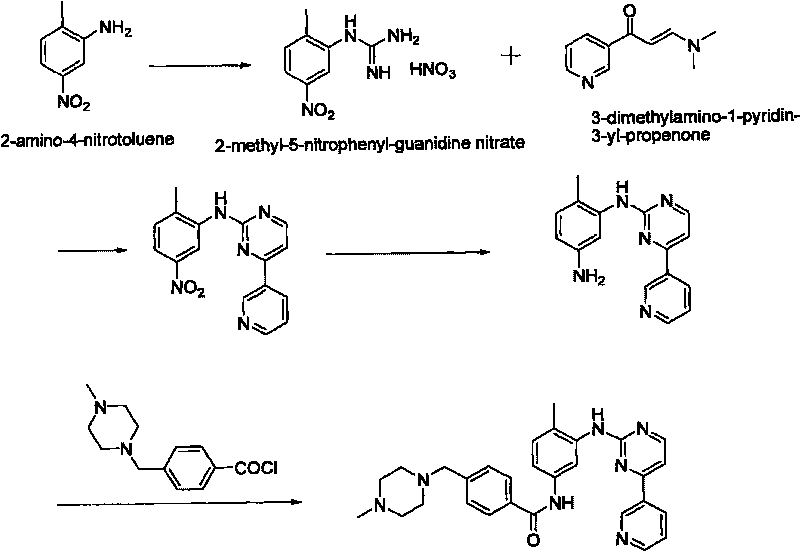
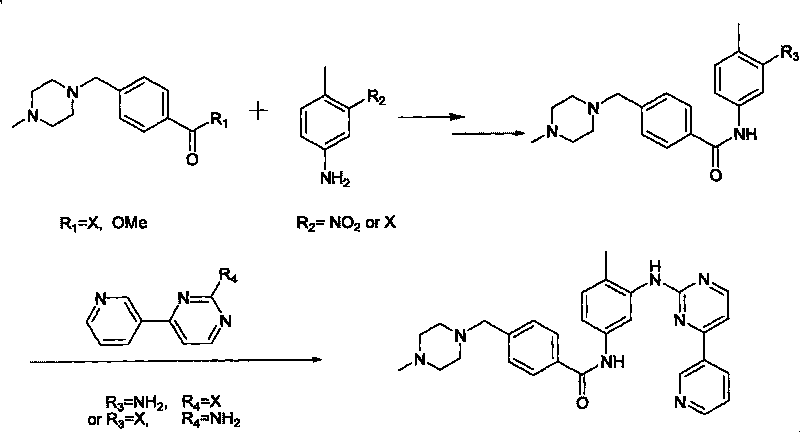
Method for synthesizing Imatinib
A synthetic method, imatinib technology, applied in the field of drug preparation, can solve the problems of unsuitability for industrial production, harsh reaction conditions, expensive raw materials, etc., and achieve the effect of being conducive to industrial production, mild conditions, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of 4-hydroxymethyl-N-(3-guanidino-4-methylphenyl)benzamide (II)
[0024] Add 0.8g (2.44mmol) 4-hydroxymethyl-N-[4-methyl-3-aminophenyl]benzamide in a 25ml four-necked flask, then add 8ml of n-butanol, heat up to reflux under stirring, Add 0.62g of concentrated hydrochloric acid dropwise, then add 1.8g (21.4mmol) of cyanamide aqueous solution (50% concentration), after reacting overnight, add sodium hydroxide to adjust to alkaline, then filter, remove the solvent in the filtrate, and perform column chromatography , and dried to obtain 0.66 g of light yellow solid. The yield was 70.2%.
[0025] Preparation of 4-hydroxymethyl-N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]phenyl]benzamide (III)
[0026] Add 15ml isopropanol, 0.7g (2.13mmol) 4-hydroxymethyl-N-(3-guanidino-4-methylphenyl) benzamide and 0.38g (2.13mmol) 3 -(3-Dimethylaminoacryl)pyridine, heated to reflux overnight with stirring, then cooled and crystallized to obtain 0.50 g of a bright yello...
Embodiment 2
[0034] Preparation of 4-hydroxymethyl-N-(3-guanidino-4-methylphenyl)benzamide (II)
[0035] Add 4g (15.6mmol) 4-hydroxymethyl-N-[4-methyl-3-aminophenyl]benzamide into a 100ml four-necked flask, then add 25ml of isopropanol, heat up to reflux under stirring, drop Add 3.1g of concentrated hydrochloric acid, then add 6.56g (78.1mmol) of cyanamide aqueous solution (50% concentration), react overnight, add sodium hydroxide to adjust to alkalinity, filter, remove the solvent in the filtrate, add a small amount of water to dissolve After cooling, crystallize slowly, and obtain 3.63 g of light yellow solid after suction filtration and drying. The yield was 78.1%.
[0036] Preparation of 4-hydroxymethyl-N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]phenyl]benzamide (III)
[0037] Add 25ml isoamyl alcohol, 5g (15.3mmol) 4-hydroxymethyl-N-(3-guanidino-4-methylphenyl) benzamide and 3.2g (18.1mmol) 3- (3-Dimethylaminoacryloyl)pyridine was heated to reflux overnight with stirring, th...
Embodiment 3
[0045] Preparation of 4-hydroxymethyl-N-(3-guanidino-4-methylphenyl)benzamide (II)
[0046] Add 10g (30.5mmol) 4-hydroxymethyl-N-[4-methyl-3-aminophenyl]benzamide into a 250ml four-necked flask, then add 62.5ml of ethanol, heat up to reflux under stirring, drop Concentrated hydrochloric acid 7.75g, then add 6.1g (76.2mmol) aqueous solution (50% concentration) of cyanamide, after reacting overnight, add sodium hydroxide to adjust to alkaline, filter, remove the solvent in the filtrate, add a small amount of water to dissolve Slowly crystallize under cooling, and obtain 9.7 g of light yellow solid after suction filtration and drying, with a yield of 80%.
[0047] Preparation of 4-hydroxymethyl-N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]phenyl]benzamide (III)
[0048] Add 45ml isoamyl alcohol, 9g (27.5mmol) 4-hydroxymethyl-N-(3-guanidino-4-methylphenyl) benzamide and 5.76g (32.7mmol) 3- (3-Dimethylaminoacryloyl)pyridine was heated to reflux under stirring, a small amount...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com