Preparation of recombinant protein a gene and its expression product
A recombinant protein and gene expression technology, applied in the field of bioengineering, can solve the problems of little influence on the activity of protein A ligand, great harm to the human body and the environment, and low activation efficiency, and achieve strong binding specificity, short expression time, and high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of embodiment 1 recombinant protein A gene monomer
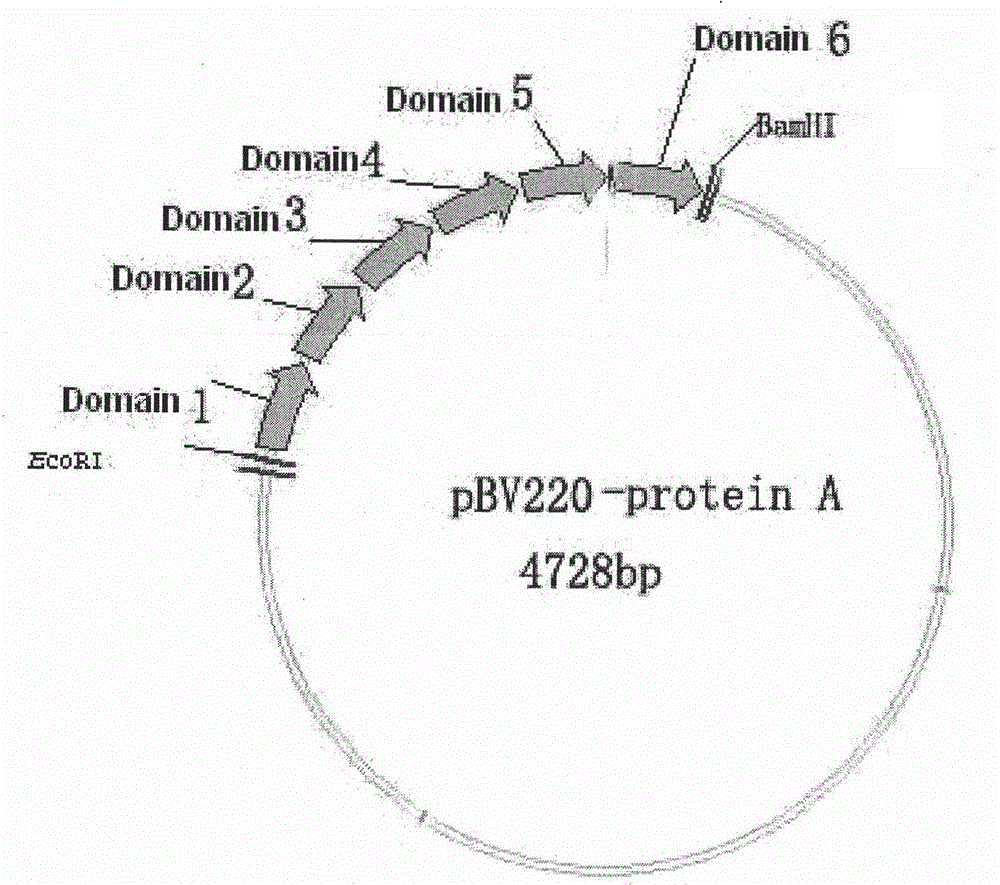
[0031] In order to promote the expression of the Staphylococcus aureus protein A gene in Escherichia coli, according to the codon preference of Escherichia coli, some base sequences in the B domain gene of protein A were optimized (there are multiple optimization schemes, sequence table SEQNo. 1 is one of the optimized sequences), through the method of chemical synthesis, the recombinant protein A gene monomer sequence was designed and synthesized. Connectors for body connection. In addition, it also includes start codon ATG, stop codon TAA, EcoRI and BamHI restriction endonuclease sites for cloning, and 6×His tag sequence at the 5′ end. Embodiment 2 Contains the construction of the pBV220 expression vector of a recombinant protein A gene monomer
Embodiment 2
[0032] The chemically synthesized recombinant protein A gene monomer was digested with EcoRI and BamHI and connected to the pBV220 vector with the same restriction enzymes, transformed into Escherichia coli, and the transformant with ampicillin resistance was screened, and the plasmid was extracted by PCR and digestion And after sequencing and identification, it was proved that the recombinant protein A gene monomer had been cloned into pBV220.
Embodiment 3
[0033] Embodiment 3 Contains the construction of the pBV220-P expression vector of recombinant protein A gene
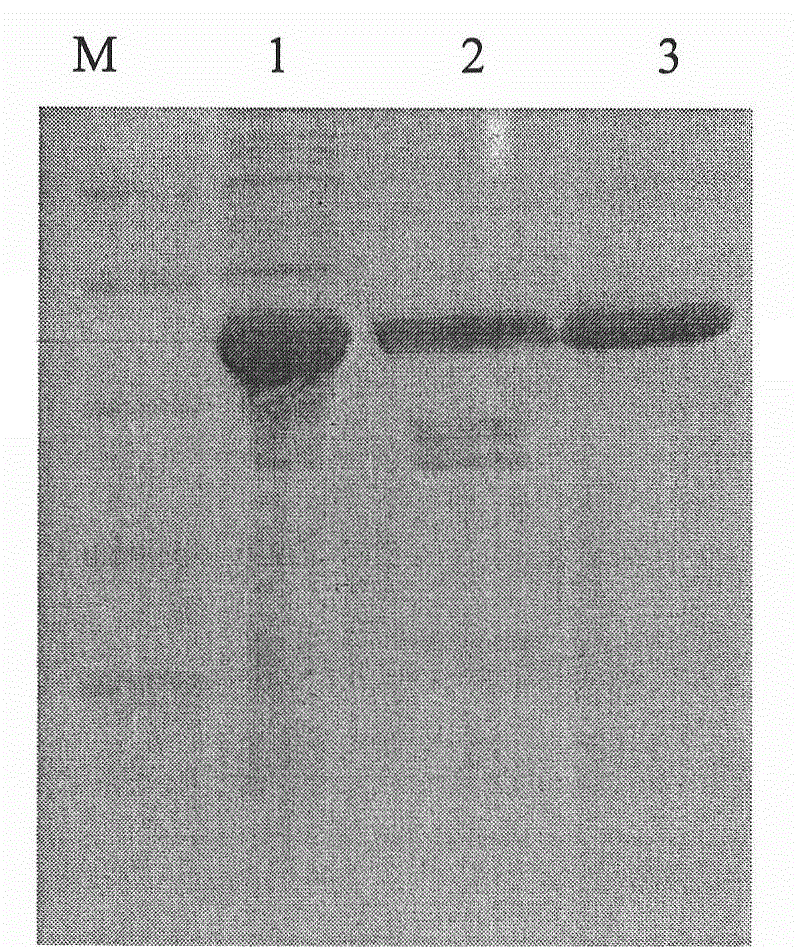
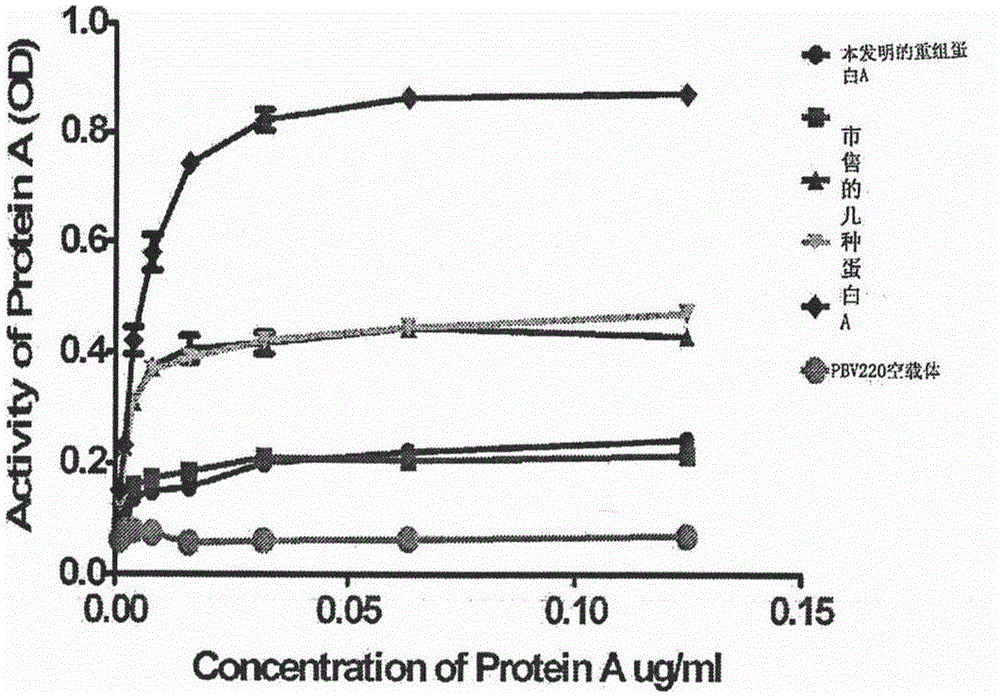
[0034] The above-mentioned pBV220 vector containing a recombinant protein A gene monomer and the recombinant protein A gene monomer were digested with AccI, and the corresponding fragments were recovered and ligated, transformed into Escherichia coli, and the transformant with ampicillin resistance was screened, and the plasmid was extracted by After identification by PCR, enzyme digestion and sequencing, it was proved that the recombinant protein A gene (six protein A gene monomers connected in series) had been cloned into pBV220. The result is attached to the manual figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com