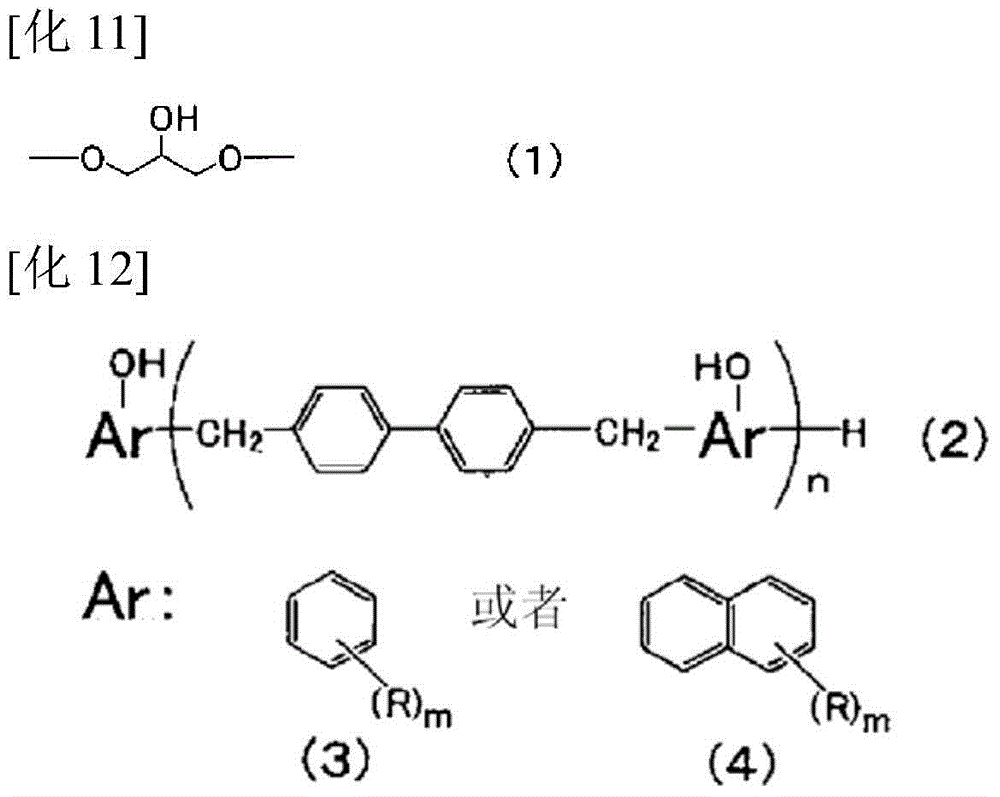
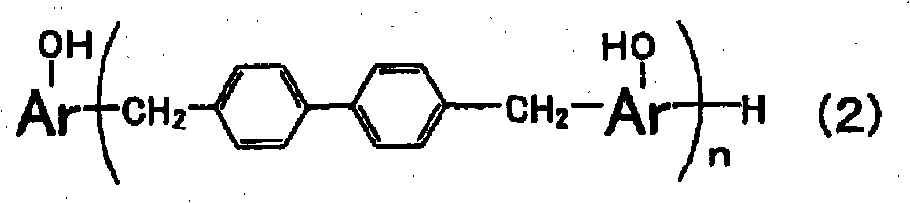
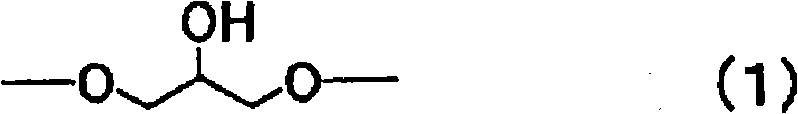Reactive carboxylate compound, curable resin composition using the reactive carboxylate compound, and use thereof
A technology of curing resins and compounds, applied in photosensitive materials for opto-mechanical equipment, photo-engraving processes for pattern surfaces, instruments, etc., can solve the problem of unbalanced, undisclosed hydroxyl and epoxy groups. and other problems, to achieve the effects of good developability, excellent resin physical properties, and high dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0193] Hereinafter, the present invention will be described in more detail by way of examples, but the present invention is not limited to these examples. In addition, unless otherwise stated in the examples, "parts" means "parts by weight".
[0194] The softening point and the epoxy equivalent were measured under the following conditions.
[0195] 1) Epoxy equivalent: measured by a method based on JISK-7236:2001.
[0196] 2) Hydroxyl equivalent: It is based on the epoxy equivalent of the corresponding epoxy resin, and according to the reaction of the epoxy group in the epoxy resin with an equivalent amount of acetic acid, after the epoxy group is ring-opened, it is based on JISK0070: 1992 Method to measure the obtained hydroxyl equivalent, and calculated.
[0197] 3) Softening point: measured by the method based on JISK-7234:1986.
[0198] 4) The measurement conditions of GPC are as follows.
[0199] Model: TOSOHHLC-8220GPC
[0200] Column: SuperHZM-N
[0201] Eluent: T...
Synthetic example 1
[0204] Synthesis Example 1: Synthesis of Epoxy Resin
[0205] Implement nitrogen purging in the flask that stirrer, reflux cooling pipe, stirring device are housed, drop into 576g phenol-biphenyl novolac type epoxy resin simultaneously ., a softening point of 70°C, a compound represented by formula (7), n≒3) as the epoxy resin of the above formula (8), the phenol-biphenyl novolac resin of the amount recorded in Table 1 (manufactured by Nippon Kayaku Co., Ltd. The KAYAHARDGPH65 hydroxyl equivalent of 197g / eq., the compound represented by the above formula (5), n≒2) is used as a phenol aralkyl resin, and then the methyl isobutyl ketone (MIBK) that makes the solid content reach 60 parts by weight is added as a solvent . After uniformly dissolving at 70°C, 0.5 g of triphenylphosphine was added, followed by stirring at 100°C for 20 hours. After the reaction is completed, oxygen purging is carried out to oxidize triphenylphosphine to obtain epoxy resin (a) resin solution.
[0206...
Synthetic example NC-300
[0209] Synthesis example NC-3000HGPH-65 (molar ratio) WPEOHVspα
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com