Reactive polyester compound, active energy ray-curable resin composition using same
A technology of active energy rays and curable resins, which is applied in the field of reactive polyester compounds and can solve the problems of low heat resistance, poor solubility and sensitivity, unsatisfactory compounds, etc., and achieve excellent resins Effects of physical properties and good developability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0123] Hereinafter, the present invention will be described in more detail using examples, but the present invention is not limited to these examples. In addition, in an Example, unless otherwise indicated, "part" means a weight part, and "%" means a weight%.
[0124] The epoxy equivalent, softening point, acid value and molecular weight were measured under the following conditions.
[0125] 1) Epoxy equivalent: according to the method of JIS K 7236:2001
[0126] 2) Softening point: According to the method of JIS K 7234:1986
[0127] 3) Acid value: According to the method of JIS K 0070:1992
[0128] 4) Molecular weight: analyzed by GPC under the following conditions
[0129] Model: TOSOH HLC-8220GPC
[0130] Column: TSKGEL Super HZM-N
[0131] Eluent: THF (tetrahydrofuran); 0.35ml per minute, 40°C
[0132] Detector: Differential refractometer
[0133] Molecular weight standard: polystyrene
Synthetic example 1
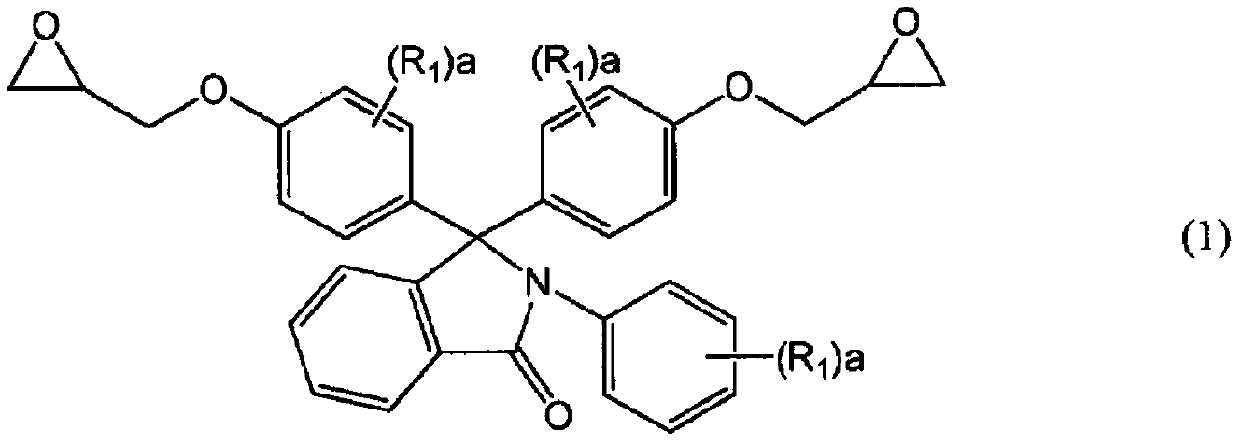
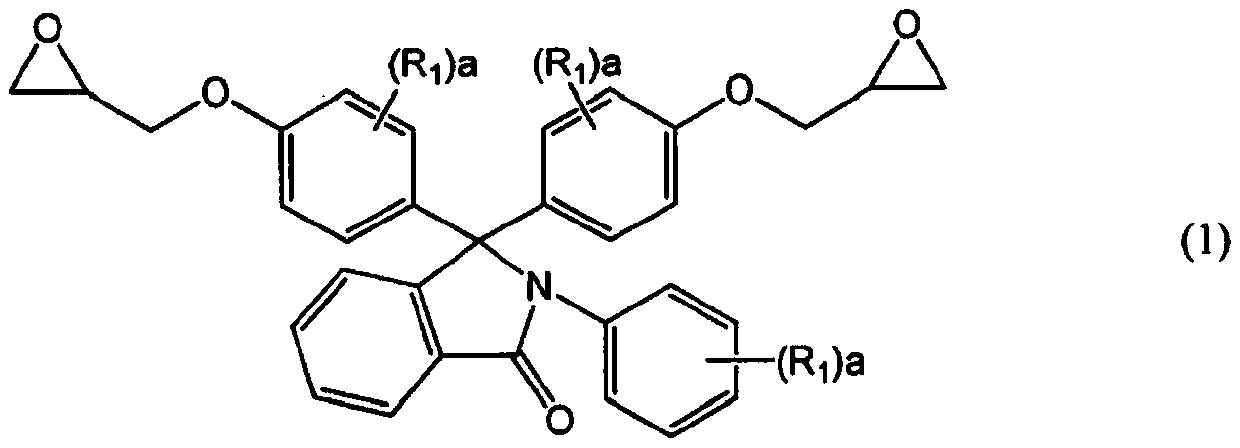
[0134] Synthetic example 1 epoxy resin (a) (R in general formula (1) 1 Synthesis of compounds with all hydrogen atoms)
[0135] According to the description in Example 1 of Patent Document 4, the following synthesis was carried out.
[0136] The flask equipped with a thermometer, a condenser, and a stirrer was purged with nitrogen, and at the same time, 256 g of N-phenylphenolphthalein (PPPBP manufactured by SABIC, with a purity of more than 99%), 842 g of epichlorohydrin, and 180 g of methanol were added as phenolic compounds. The temperature was raised to 75°C. When the internal temperature exceeded 65° C., 21 g of flaky sodium hydroxide was added in portions over 90 minutes, and the reaction was further performed at 70° C. for 1 hour. After the reaction, wash twice with 300 g of water to remove the generated salts, and then remove excess epichlorohydrin and the like while stirring under reduced pressure (~70°C, -0.08MPa~-0.09MPa) for 3 hours. Distilled off. To the resid...
Embodiment 1
[0137] Embodiment 1: the synthesis of reactive polyester compound (A-1)
[0138] In a 2L flask equipped with a stirring device and a reflux tube, the epoxy resin (N-phenylphenolphthalein type epoxy resin) synthesized in Synthesis Example 1 was added as the epoxy resin (a) having two or more epoxy groups in the molecule. Resin, epoxy equivalent: 266g / equivalent) 266g, propylene glycol monomethyl ether monoacetate as the solvent for reaction make solid content be 70%, drop in as the monobasic carboxylic acid compound ( b) 72.1 g of acrylic acid (abbreviated as AA, Mw=72), 1.01 g of triphenylphosphine as a catalyst, and 0.17 g of 2-methylhydroquinone as a polymerization inhibitor are reacted at a temperature of 98° C. The acid value became 1.0 mg·KOH / g or less to obtain a diol compound (theoretical molecular weight: 649.69).
[0139] Next, propylene glycol monomethyl ether monoacetate as a solvent was added to the glycol compound solution thus obtained so that the solid content ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com