Reactive epoxy carboxylate compound, resin composition containing the compound, and cured product of the resin composition
A technology of epoxy carboxylate and resin composition, which is applied in the photoplate making process of the pattern surface, photosensitive materials used in optical mechanical equipment, instruments, etc., can solve the problem of false agglutination of dispersion liquid, poor stability, Unknown problems such as acid-modified epoxy acrylate compounds, etc., to achieve high temperature and high humidity and thermal shock resistance, good storage stability, and excellent resin physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
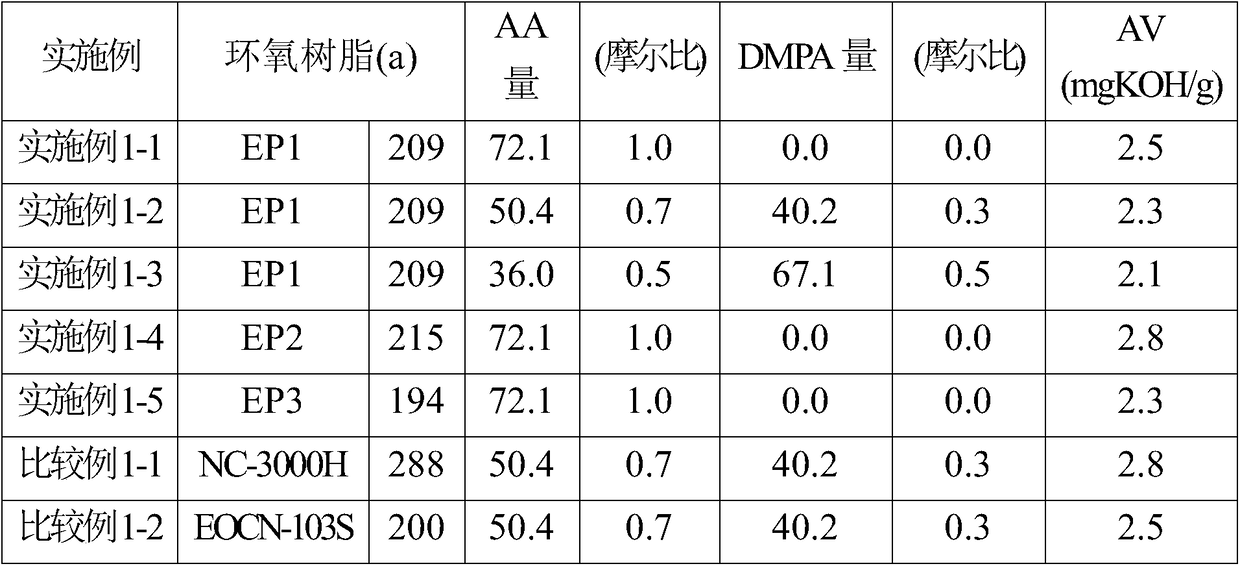
Examples
Synthetic example 1
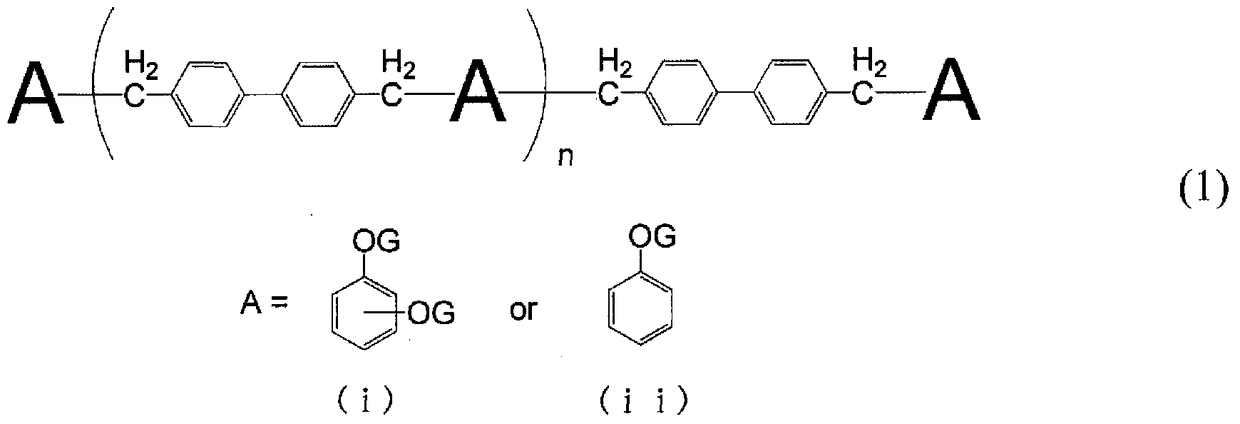
[0126] Add 316 parts of phenol and 158 parts of resorcinol to a flask with a thermometer, a cooling tube, and a stirrer, and after raising the temperature to 100°C, add 4,4'-bischloromethylbiphenyl 201 in batches over 2 hours Parts were reacted at the same temperature for another 5 hours. Thereafter, the temperature was raised to 160°C to react all the 4,4'-bischloromethylbiphenyl groups. During this time, generated HCl was distilled off with a basic separator. After completion of the reaction, 266 parts of phenol resins (P-1) were obtained by distilling off unreacted phenol and unreacted resorcinol at 180° C. under reduced pressure using a rotary evaporator. The obtained phenol resin (P-1) had a hydroxyl equivalent of 137 g / eq., a softening point of 94° C., an ICI viscosity of 470 mPa·s, and a divalent phenol introduction ratio of 64%.
Synthetic example 2
[0128] Add 266 parts of phenol resin obtained in Synthesis Example 1, 719 parts of epichlorohydrin, 72 parts of methanol and 21 parts of water to a flask equipped with a stirrer, a reflux cooling tube, and a stirring device while performing nitrogen purge, and heat up to 75°C . Next, after adding 83 parts of flake-shaped sodium hydroxide in batches over 90 minutes, the reaction was further performed at 75° C. for 75 minutes. After completion of the reaction, water washing was performed, and solvents such as excess epichlorohydrin were distilled off from the organic layer under reduced pressure at 140° C. using a rotary evaporator. To the residue, 750 parts of methyl isobutyl ketone was added and dissolved, and the temperature was raised to 75°C. After adding 52 parts of 30% sodium hydroxide aqueous solution with stirring, and reacting for 1 hour, the organic layer was washed with water until the washing water became neutral, and the obtained organic layer was removed from the...
Synthetic example 3
[0130] Add 316 parts of phenol and 126 parts of resorcinol to a flask with a thermometer, a cooling tube, and a stirrer, and after raising the temperature to 100°C, add 201 parts of 4,4'-bischloromethylbiphenyl in batches over 2 hours The base was reacted at the same temperature for another 5 hours. Thereafter, the temperature was raised to 160°C to react all the 4,4'-bischloromethylbiphenyl groups. During this time, generated HCl was distilled off with a basic separator. After completion of the reaction, 194 parts of phenol resins (P-2) were obtained by distilling off unreacted phenol and unreacted resorcinol at 180° C. under reduced pressure using a rotary evaporator. The obtained phenol resin (P-2) had a hydroxyl equivalent of 141 g / eq., a softening point of 89° C., an ICI viscosity of 446 mPa·s, and a divalent phenol introduction ratio of 51%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com