Preparation method of lactone polymer, polyurethane and polyester
A polymer and polyurethane technology, applied in the field of preparation of lactone polymers, can solve the problems of unsatisfactory, high color number, high viscosity, etc., and achieve the effects of excellent heat resistance, low viscosity and low color number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
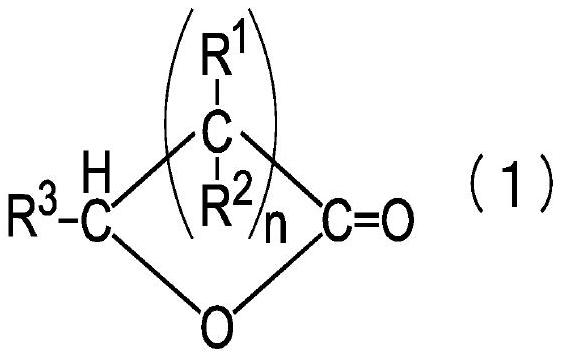
[0025] The present invention relates to a method for producing a lactone polymer obtained by subjecting a lactone compound represented by the general formula (1) to ring-opening polymerization. In addition, the preparation method of the present invention relates to a preparation method of a lactone polymer, which is characterized in that a non-metallic halide or a heteropolyacid is used as a catalyst, and an active hydrogen atom-containing compound is used as an initiator in the ring-opening polymerization. compound, adjusting the moisture content of the compound containing active hydrogen atoms.
[0026] As the catalyst in the production method of the lactone polymer of the present invention, a non-metallic halide can be exemplified as a Lewis acid, or a heteropolyacid can be exemplified as a solid acid. As non-metallic halides, boron trifluoride (BF 3 ), phosphorus pentafluoride (PF 5 ) and other non-metallic halides. As a heteropolyacid, phosphotungstic acid hydrate (H ...
Embodiment 1
[0062] [Example 1] Synthesis of polycaprolactone
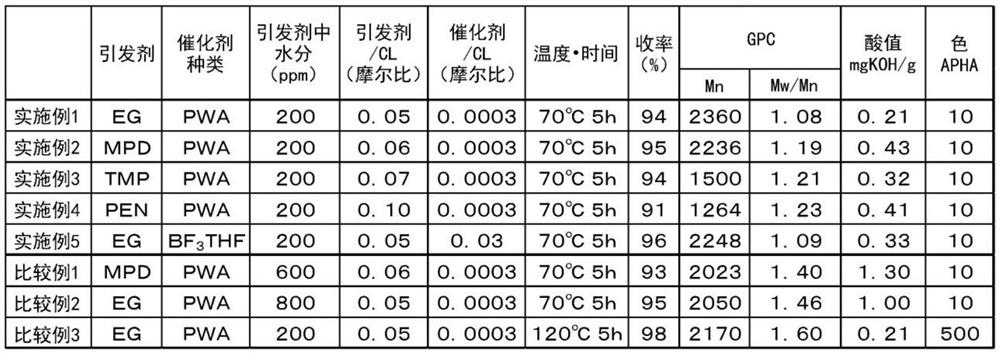
[0063]Into a 500 mL reaction vessel purged with nitrogen, ε-caprolactone (abbreviated as CL) 114.0 g (1.00 mol, manufactured by Tokyo Chemical Industry Co., Ltd.), ethylene glycol (EG) 3.2 g (0.05 mol, moisture content 200 ppm , manufactured by Kishida Chemical Co., Ltd.), and 1.0 g of phosphotungstic acid hydrate (PWA) (0.0003 mol, manufactured by Nippon Shinkoku Co., Ltd.) was added, and stirred at 70° C. for 5 hours. 100 mL of toluene and 50 g of ion exchange resin IRA-96SB (manufactured by Japan Organo Co., Ltd.) were added to the reaction mixture, followed by stirring at room temperature for 2 hours. After stirring, the reaction mixture was filtered, and the solvent was removed from the filtrate by distillation under reduced pressure, whereby a polymer (107 g, yield 94%) was obtained as a colorless solid (normal temperature). The GPC measurement result (Mn, Mn) of the obtained lactone polymer (polycaprolactone) W / M n ...
Embodiment 2
[0064] [Example 2] Synthesis of polycaprolactone
[0065] Except that the ethylene glycol in Example 1 was replaced by 2-methyl-1,3-propanediol (MPD) 5.0g (0.06mol, moisture content 200ppm, manufactured by Kishida Chemical Co., Ltd.), through the same method as in Example 1 A colorless solid (room temperature) lactone polymer (108 g, yield 95%) was obtained. Table 1 summarizes the analysis results of the obtained polymer measured by the same method as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com