Reactive carboxylate compound, active-energy-ray-curable resin composition utilizing the same, and use of the same
A technology of active energy rays and curable resins, which is applied in the direction of photosensitive materials used in optomechanical equipment, photoplate-making process of pattern surface, instruments, etc., can solve problems such as poor stability and false coagulation of pigment dispersion liquid, and achieve Excellent resin properties, good pigment dispersibility, and high developability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
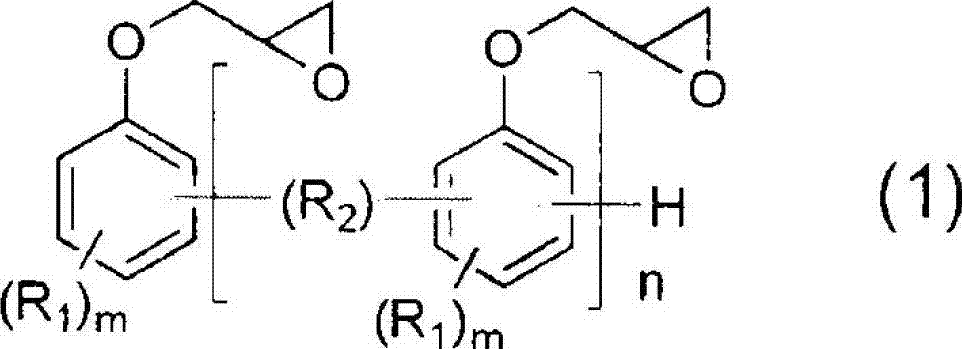
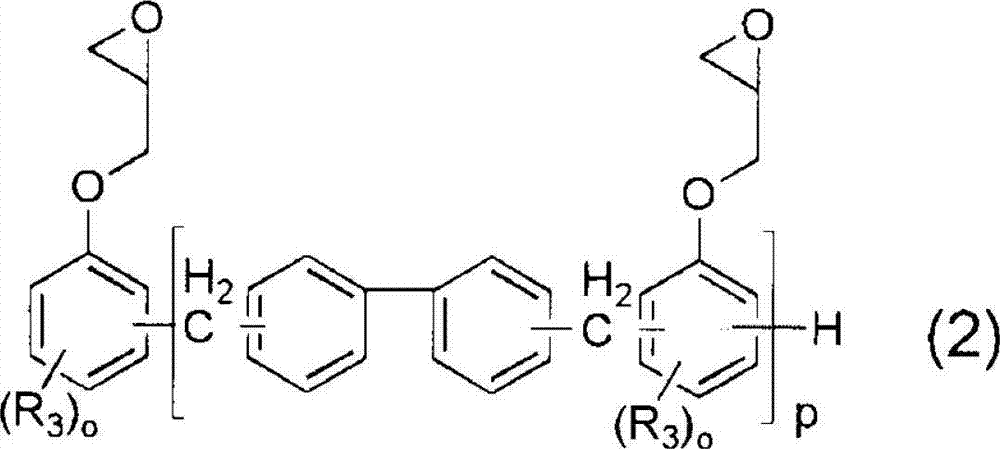
[0166] Embodiment 1: Utilize the epoxy resin shown in general formula (2) to prepare carboxylate compound (A)
[0167] Add 288g NC-3000H (manufactured by Nippon Kayaku, softening point 70°C, epoxy equivalent 288g / eq, R given in general formula (2) 3 All are hydrogen atoms, and p is 3 as an average value) as epoxy resin (a), acrylic acid (abbreviated as AA, Mw=72) in the amount recorded in Table 1 as having one or more polymerizable ethylenic bonds in the molecule Compound (b) with unsaturated group and more than one carboxyl group, dimethylol propionic acid (DMPA for short, Mw=134) in the amount recorded in Table 1 as the compound (c) with more than one hydroxyl group and more than one carboxyl group in the molecule ). The equivalent ratios of AA and DMPA charged to epoxy groups are described in molar ratios.
[0168] 3 g of triphenylphosphine was added as a catalyst, and propylene glycol monomethyl ether monoacetate having a solid content of 80 mass % was added as a solvent...
Embodiment 2
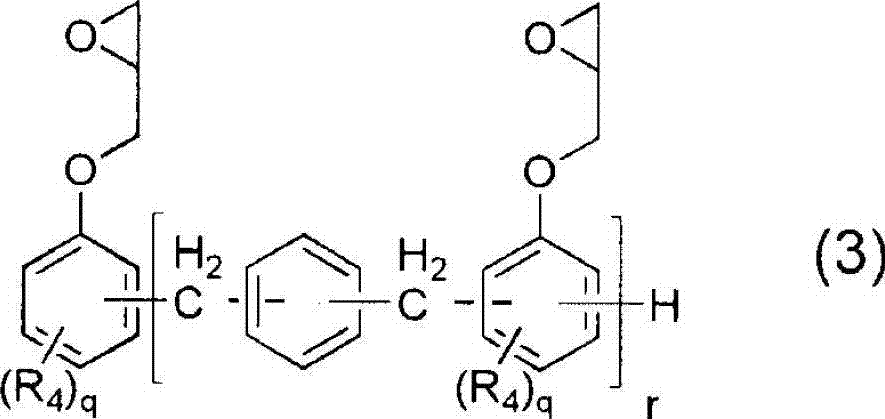
[0170] Embodiment 2: Utilize the epoxy resin shown in general formula (3) to prepare carboxylate compound (A)
[0171] Add 230g NC-2000 (manufactured by Nippon Kayaku, softening point 60°C, epoxy equivalent 230g / eq, R given in general formula (3) 4 All are hydrogen atoms, r is an average value of 5) as epoxy resin (a), acrylic acid in the amount recorded in Table 1 as compound (b) and dimethylolpropionic acid in the amount recorded in Table 1 as compound (c). The equivalent ratios of AA and DMPA charged to epoxy groups are described in molar ratios.
[0172] 3 g of triphenylphosphine was added as a catalyst, and propylene glycol monomethyl ether monoacetate so that the solid content became 80 mass % of the reaction liquid was added as a solvent, and it was made to react at 100 degreeC for 24 hours, and the carboxylate compound (A) solution was obtained.
[0173] The reaction end point was determined by solid content acid value (AV; mgKOH / g), and the measured values are des...
Embodiment 3
[0174] Embodiment 3: utilize the epoxy resin shown in general formula (4) to manufacture epoxy carboxylate compound (A)
[0175] Add 250g XD-1000 (manufactured by Nippon Kayaku, 70°C of softening point, epoxy equivalent 250g / eq, R given in general formula (4) 5 All are hydrogen atoms, t is 3 as an average value) as epoxy resin (a), acrylic acid in the amount described in Table 1 as compound (b) and dimethylolpropionic acid in the amount described in Table 1 as compound (c). The equivalent ratios of AA and DMPA charged to epoxy groups are described in molar ratios.
[0176] Using 3 g of triphenylphosphine as a catalyst, propylene glycol monomethyl ether monoacetate to make the solid content 80 mass % was added as a solvent, it was made to react at 100 degreeC for 24 hours, and the epoxy carboxylate compound (A) solution was obtained.
[0177] The reaction end point was determined by solid content acid value (AV; mgKOH / g), and the measured values are described in Table 1. F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com