CSF-1R inhibitors for treatment of cancer and bone diseases
A CSF-1R, disease technology, applied in bone diseases, urinary system diseases, cardiovascular system diseases, etc., can solve problems such as difficult design of selective inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
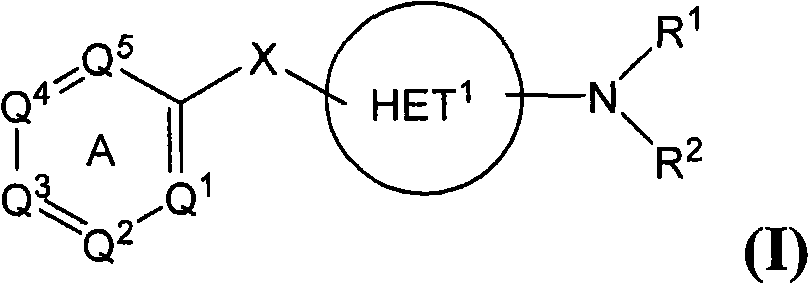
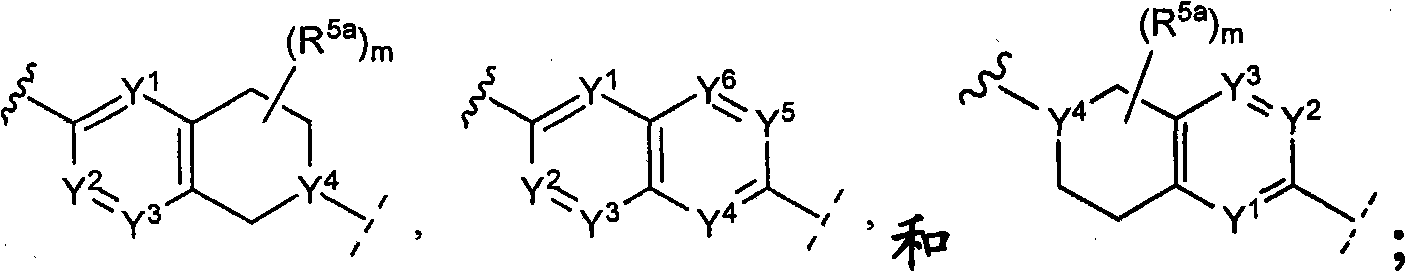
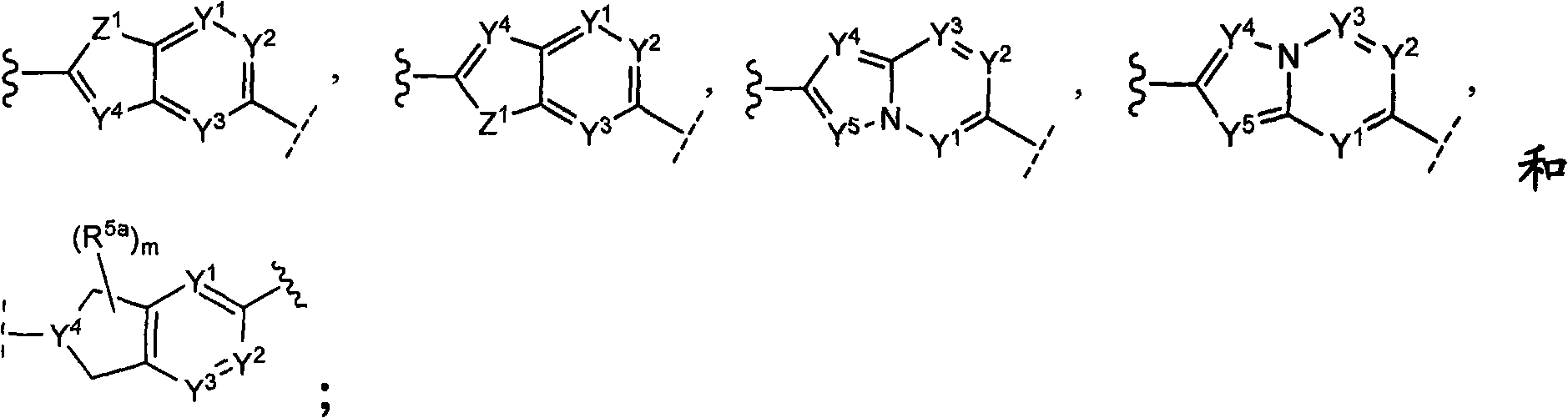
Image
Examples
Embodiment 1
[0482] 4-(2-((1R,2R)-2-hydroxycyclohexylamino)quinolin-6-yloxy)-N-methylpicolinamide
[0483]
[0484] The target compounds were prepared according to the general scheme below:
[0485] step 1
[0486]
[0487] step 2
[0488]
[0489] step 3
[0490]
[0491] Preparation of step 1.4-(2-hydroxyquinolin-6-yloxy)-N-methylpicolinamide
[0492] To a solution of 2,6-quinolinediol (500 mg, 3.10 mmol, 1.0 eq) in 10 mL of NMP was added Cs at room temperature 2 CO 3 (2.52g, 7.75mmol, 2.5eq.) and 4-chloro-N-methylpicolinamide (632mg, 3.72mmol, 1.2eq.), the reaction was stirred at 80°C for about 12 hours. Then, the reaction was quenched with water (about 100 mL) and extracted with EtOAc (3 x 100 mL). The combined organic phases were dried over sodium sulfate and concentrated in vacuo to give the title compound which was used in the next step without further purification. M+H=[296]
[0493] Step 2. Preparation of 4-(2-chloroquinolin-6-yloxy)-N-methylpicolinamide
[04...
Embodiment 2
[0498] 4-(2-((1R,2R)-2-hydroxycyclohexylamino)quinazolin-6-yloxy)-N-methylpyridinamide
[0499]
[0500] The target compounds were prepared according to the general scheme below:
[0501] Step 1.2-(Methylthio)quinazolin-6-ol
[0502]
[0503] To a solution of 2-chloro-6-methoxyquinazoline (500mg, 2.56mmol) in DMF was added NaSMe (630mg, 8.99mmol, 3.5eq) and the reaction was heated at 80°C for about 16 hours. Subsequently, the reaction was quenched with water (about 100 mL). The aqueous layer was neutralized with a few drops of HCl and extracted with EtOAc (3 x 100 mL). The combined organic phases were dried over sodium sulfate and concentrated in vacuo to give the title compound which was used in the next step without further purification. M+H=[193.1]
[0504] Step 2. Preparation of N-methyl-4-(2-(methylthio)quinazolin-6-yloxy)pyridineamide
[0505]
[0506] Cs was added to a solution of 2-(methylthio)quinazolin-6-ol (250mg, 1.30mmol, 1.0eq) in 10mL of NMP at roo...
Embodiment 3
[0514] 4-(2-(cyclohexylmethylamino)quinazolin-6-yloxy)-N-methylpyridinamide
[0515]
[0516] The title compounds were prepared according to the general scheme below.
[0517] Step 1. Preparation of N-methyl-4-(2-(methylsulfonyl)quinazolin-6-yloxy)pyridineamide
[0518]
[0519] At 0° C., to a solution of N-methyl-4-(2-(methylthio)quinazolin-6-yloxy)pyridineamide (1.0 g, 3.06 mmol, 1.0 eq) in 40.5 mL of DCM was added 4.05 mLAcOH (10%) and the mixture was stirred for 15 minutes. Then mCPBA (1.59 g, 9.20 mmol, 3.0 eq) was added in small portions, and the reaction was stirred at room temperature for 1 hour. The reaction was quenched with water (100 mL) and extracted with more DCM (3 x 100 mL). The combined organic phases were washed with saturated sodium bicarbonate solution (1 x 100 mL) and brine (1 x 100 mL), dried over sodium sulfate and concentrated in vacuo to give the title compound as a white solid which was used directly in the next step without further purificat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com