Electrolyte solution
A technology of electrolyte and electrolyte salt, applied in the field of electrolyte
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0385] Mix component (A) / component (B) / component (C1) / component (C2) at a volume % ratio of 40 / 5 / 10 / 45 to prepare a solvent for dissolving electrolyte salt, and add to the solvent for dissolving electrolyte salt Component (IIb) was adjusted to a concentration of 1 mol / liter, and stirred well at 25° C. to produce the electrolytic solution of the present invention.
Embodiment 2~35
[0387] The electrolytic solution of the present invention was produced in the same manner as in Example 1, except that components (A) to (G) and electrolyte salt (II) were changed to those described in Tables 1 to 5.
Embodiment 36~40
[0454] Examples 36-40 and Comparative Example 2
[0455] The electrolytic solution of the present invention was produced in the same manner as in Example 1, except that components (A) to (G) and electrolyte salt (II) were changed to those described in Table 6.
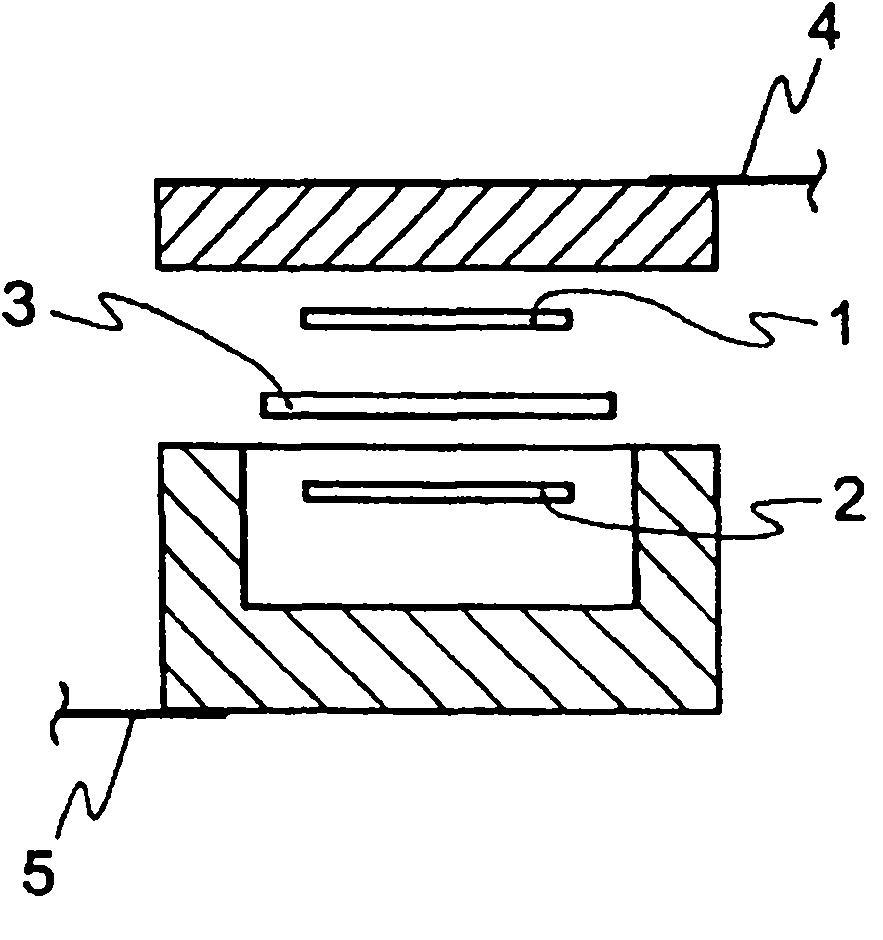
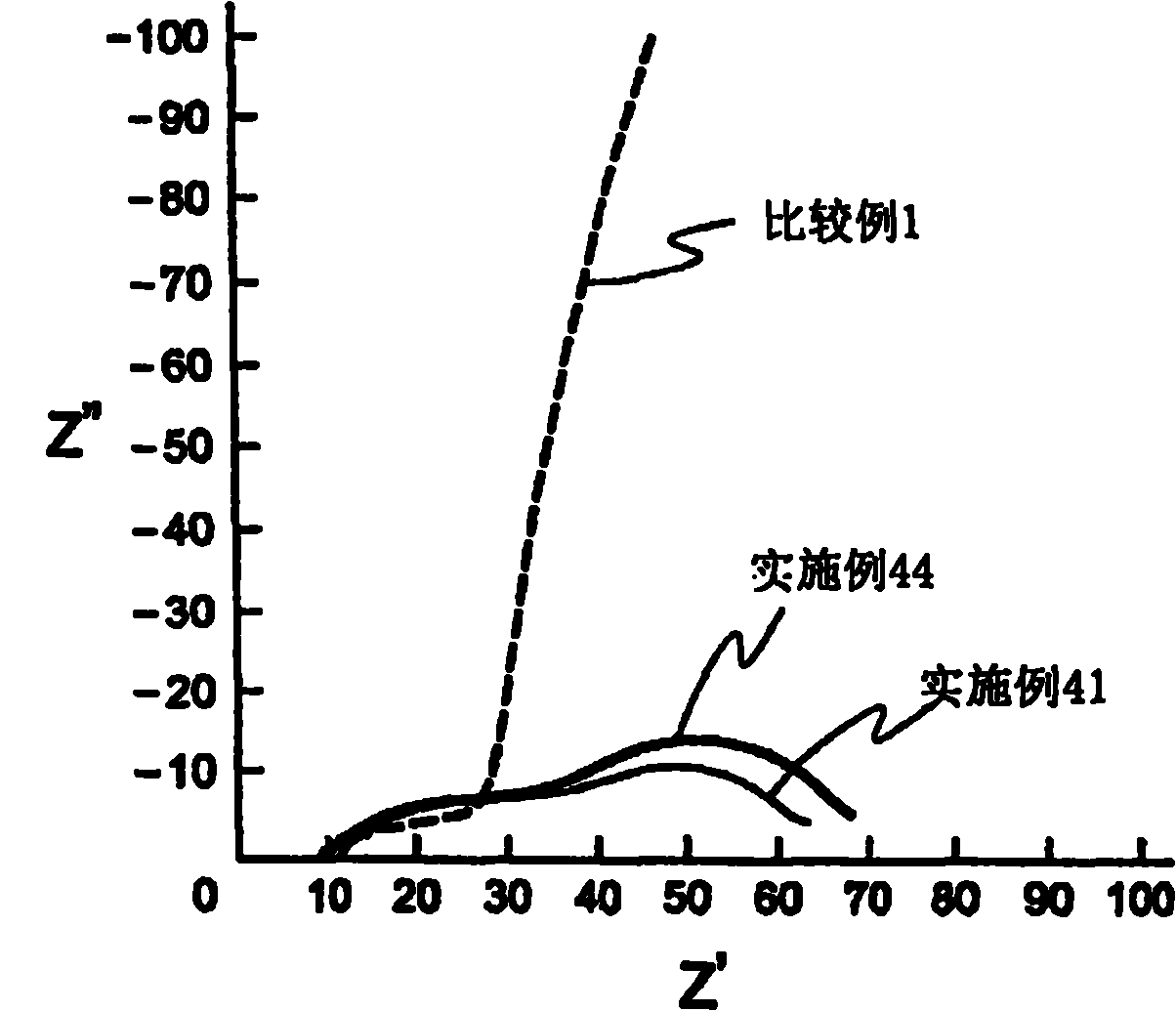
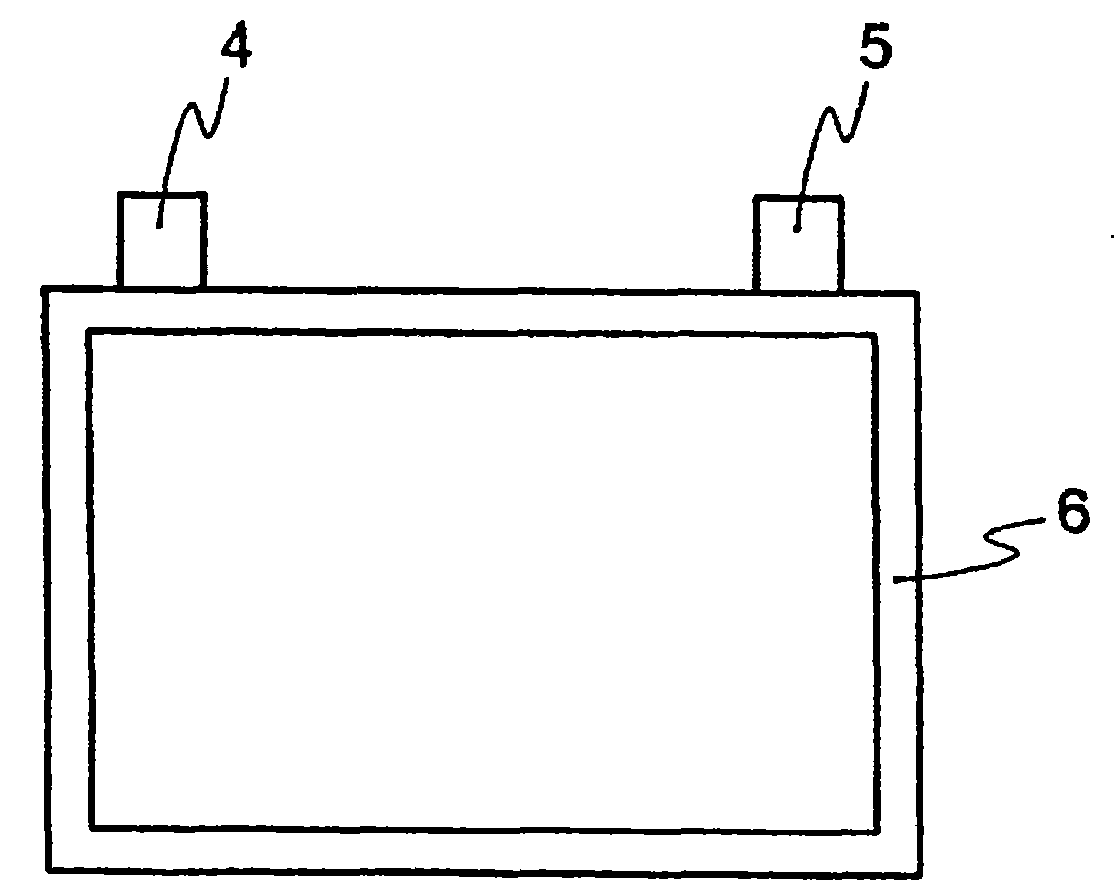
[0456] For these electrolytic solutions, solubility of electrolyte salt, low temperature stability, charge and discharge characteristics (button), and flame retardancy tests (nail penetration test, overcharge test, short circuit test) were performed. Table 6 shows the results.
[0457] [Table 6]
[0458] Table 6
[0459]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| ignition point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com