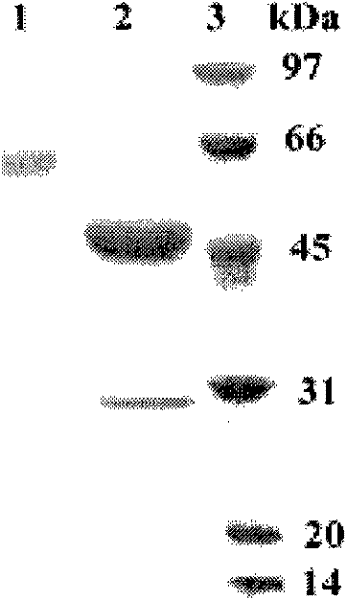
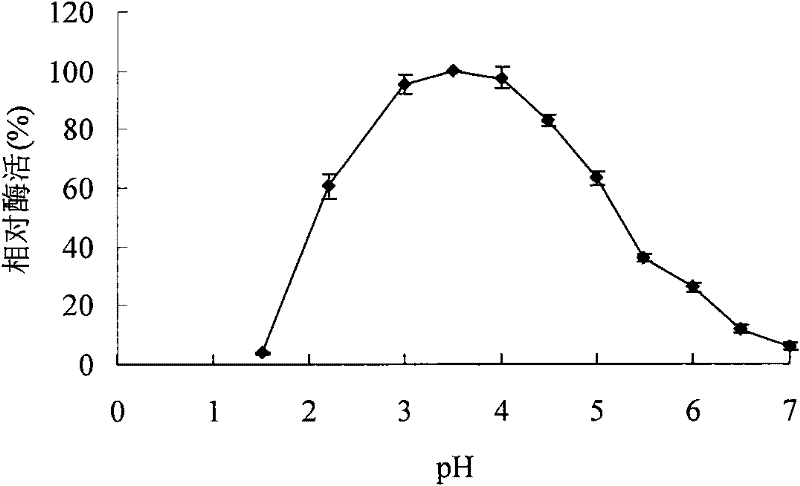
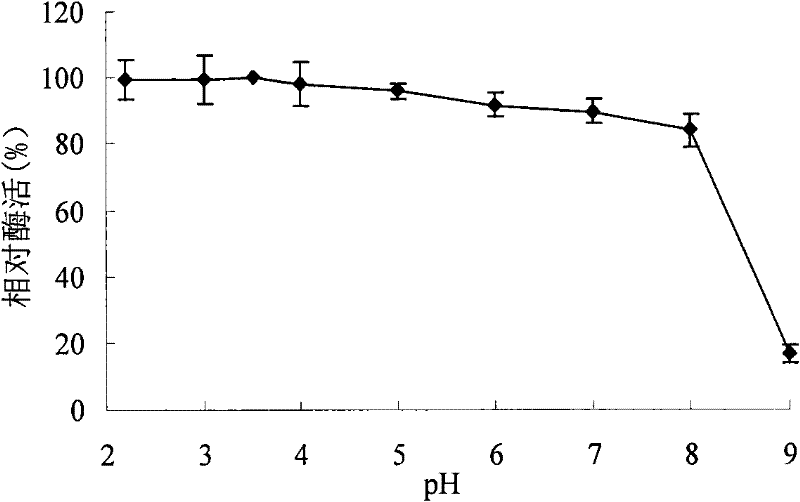
A kind of acidophilic α-galactosidase agalb with galactomannan degrading ability and its gene and application
A technology of galactomannan and galactosidase, which is applied in the field of genetic engineering and can solve problems such as inability to hydrolyze various substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Cloning of the gene AgalB encoding the acidophilic fungus Bispora sp.MEY-1α-galactosidase
[0046] Extraction of acidophilic fungus Bispora sp.MEY-1 genomic DNA:
[0047] Filter the mycelium cultured in liquid for 3 days with sterile filter paper, put it into a mortar, add 2mL of extract, grind for 5min, then put the grinding solution in a 50mL centrifuge tube, lyse in a water bath at 65°C for 20min, and mix every 10min. Homogenize once and centrifuge at 10,000 rpm for 5 min at 4°C. The supernatant was extracted in phenol / chloroform to remove impurity proteins, and then an equal volume of isopropanol was added to the supernatant. After standing at room temperature for 5 minutes, centrifuge at 10,000 rpm for 10 minutes at 4°C. The supernatant was discarded, the precipitate was washed twice with 70% ethanol, dried in vacuum, dissolved by adding an appropriate amount of TE, and stored at -20°C for later use.
[0048] Through the construction of the cDNA library...
Embodiment 2
[0052] RT-PCR analysis of embodiment 2α-galactosidase gene
[0053] Extract the total RNA of Bispora sp.MEY-1, use reverse transcriptase to obtain a strand of cDNA, and then design appropriate primers (AgalB F1: 5′-ATGGCGATACTTTTTGCTTGCTTTGCGACGCTTG-3′, AgalB R: 5′-TCATACCGAACATTGATAGTAGAAGGCGCATGTGC-3′) The single-stranded cDNA was amplified to obtain the cDNA sequence of α-galactosidase, and the amplified product was recovered and sent to Shanghai Sangong for sequencing.
[0054] By comparing the genome sequence and cDNA sequence of α-galactosidase, it is found that the gene has 3 introns, the cDNA is 1281bp long, encodes 426 amino acids and a stop codon, and the N-terminal 17 amino acids are its predicted signal peptide Sequence, the measured nucleotide sequence of the mature protein part of the gene AgalB is homologously compared with the α-galactosidase gene sequence on GeneBank, the highest amino acid sequence identity is 35%, and the nucleotide sequence identity is 36% ...
Embodiment 3
[0055] Example 3 Preparation of recombinant α-galactosidase.
[0056] The expression vector pPIC9 was subjected to double digestion (SnaBI+NotI), and the gene AgalB encoding α-galactosidase was double-digested (SnaBI+NotI) at the same time, and the gene fragment encoding mature α-galactosidase was cut out and expressed The vector pPIC9 was ligated to obtain the recombinant plasmid pPIC-AgalB containing the Bispora sp.MEY-1α-galactosidase gene AgalB and transformed into Pichia pastoris GS115 to obtain the recombinant Pichia pastoris strain GS115 / AgalB.
[0057] The GS115 strain containing the recombinant plasmid was inoculated in 400mL of BMGY culture medium, shaken at 250rpm at 30°C for 48h, and then collected by centrifugation. Then resuspend in 200mL BMMY medium, shake culture at 20°C and 250rpm. After 72 hours of induction, the supernatant was collected by centrifugation. The activity of α-galactosidase was determined. The expression level of recombinant α-galactosidase ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com