Photosensitive graft polymer, and photosensitive resin composition comprising the same
A technology of grafted polymers and polymeric compounds, which is applied in the field of photosensitive resin compositions, can solve the problems that the dispersion stability of pigments cannot meet the needs, and achieve the effects of excellent transparency and excellent dispersion stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
Example
Hereinafter, examples and comparative examples are shown to specifically explain the present invention, but the present invention is not limited to these examples.
Example Embodiment
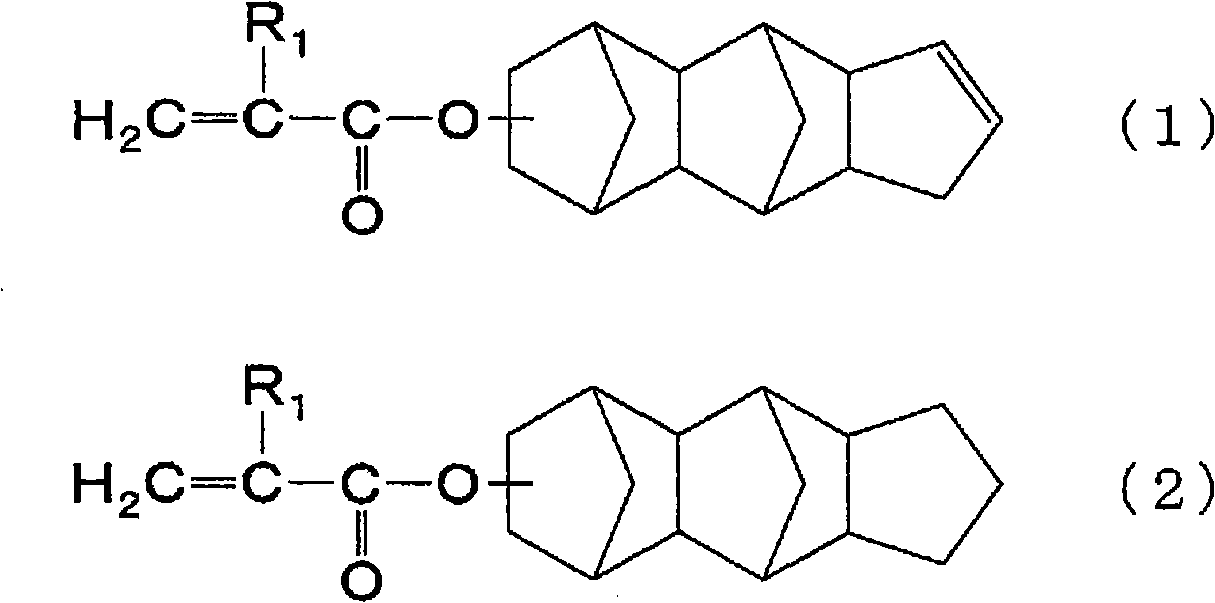
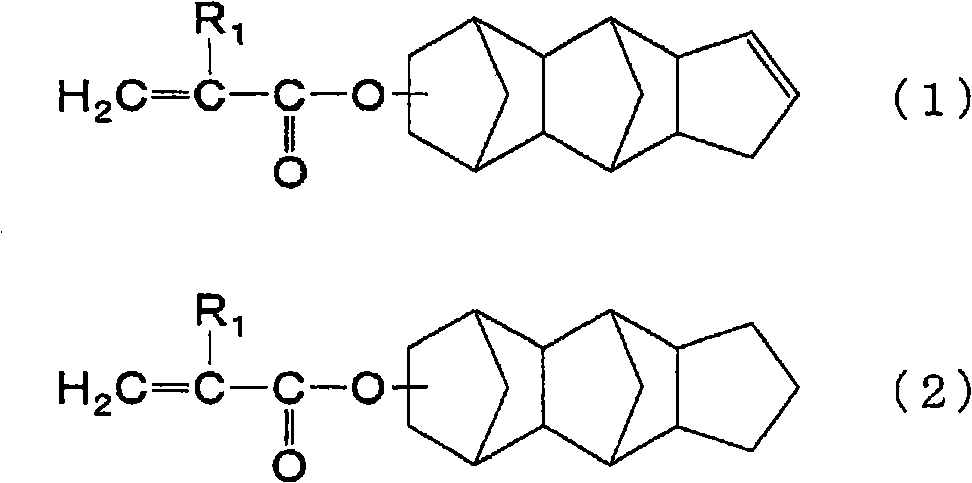
In a flask equipped with a stirring device, a dropping funnel, a condenser, a thermometer, and an introduction tube, 46.2 parts by mass of propylene glycol monomethyl ether acetate and tricyclo[5.2.1.02, 6] dec-8-yl methacrylate were added 11.1 parts by mass (approximately 0.050 mol) and 8.9 parts by mass (approximately 0.051 mol) of benzyl methacrylate were stirred and heated to 120°C while replacing with nitrogen. Next, 44.4 parts by mass (about 0.202 mol) of tricyclo[5.2.1.02,6]dec-8-yl methacrylate, 35.6 parts by mass (about 0.202 mol) of benzyl methacrylate, and 1.9 parts by mass of thioglycolic acid A solution of 6.0 parts by mass of tert-butyl peroxide (2-ethylhexanoic acid) was added dropwise to the flask from the dropping funnel over 2 hours, and then reacted at 120°C for 0.5 hours.
Next, the inside of the flask was replaced with air, and then 2.9 parts by mass of glycidyl methacrylate, 0.3 parts by mass of triphenylphosphine, and 0.06 parts by mass of methylhydroqui...
Example Embodiment
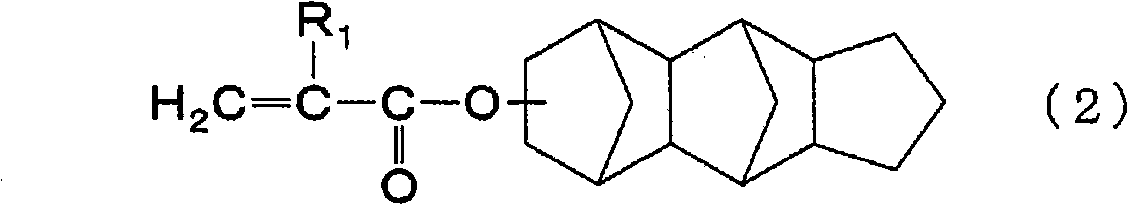
In a flask equipped with a stirring device, a dropping funnel, a condenser, a thermometer, and an introduction tube, 46.2 parts by mass of propylene glycol monomethyl ether acetate and tricyclo[5.2.1.02, 6] dec-8-yl methacrylate were added 18.4 parts by mass (approximately 0.084 mol) and 1.6 parts by mass (approximately 0.009 mol) of benzyl methacrylate were stirred and heated to 120°C while replacing with nitrogen. Next, 73.5 parts by mass (about 0.334 mol) of tricyclo[5.2.1.02,6]dec-8-yl methacrylate, 6.5 parts by mass (about 0.037 mol) of benzyl methacrylate, and 1.7 parts by mass of thioglycolic acid A solution of 6.0 parts by mass of tert-butyl peroxide (2-ethylhexanoic acid) was added dropwise to the flask from the dropping funnel over 2 hours, and then reacted at 120°C for 0.5 hours.
Next, the inside of the flask was replaced with air, and then 2.9 parts by mass of glycidyl methacrylate, 0.3 parts by mass of triphenylphosphine, and 0.06 parts by mass of methylhydroquino...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solid content acid value | aaaaa | aaaaa |
| Solid content acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com