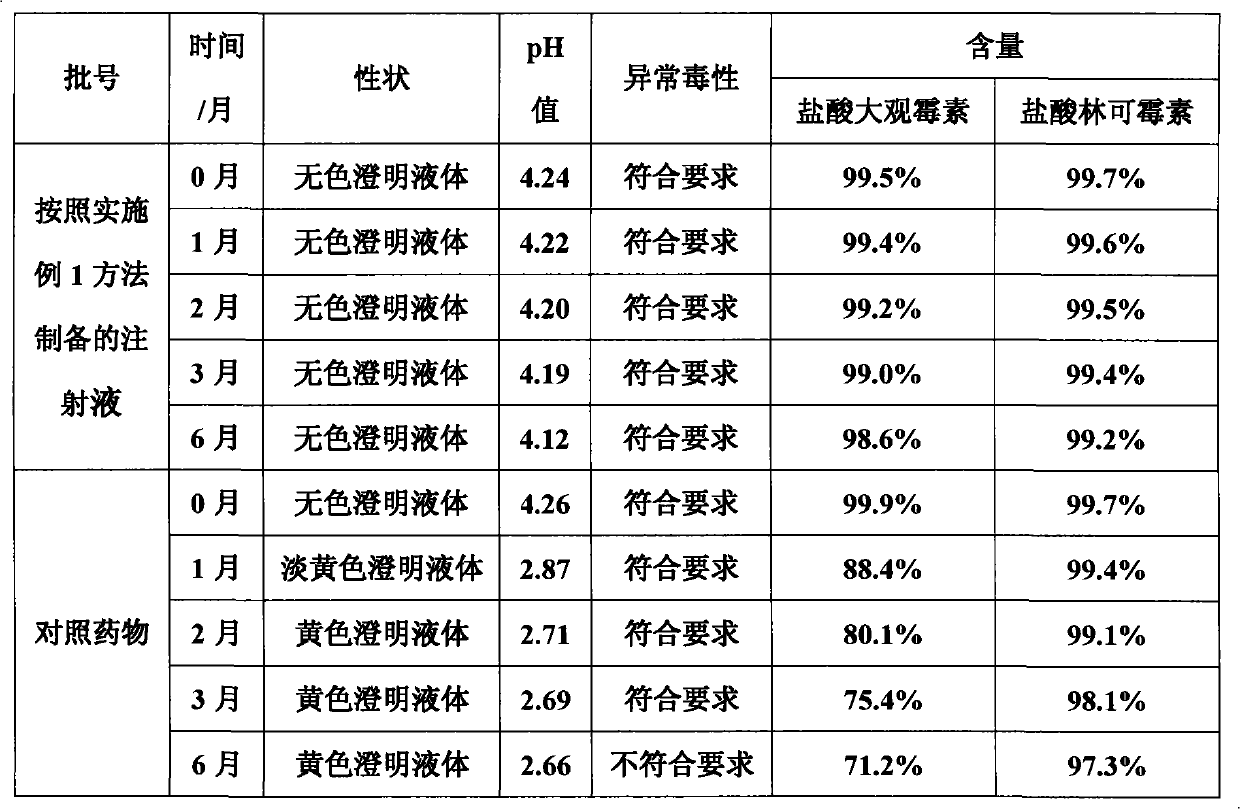
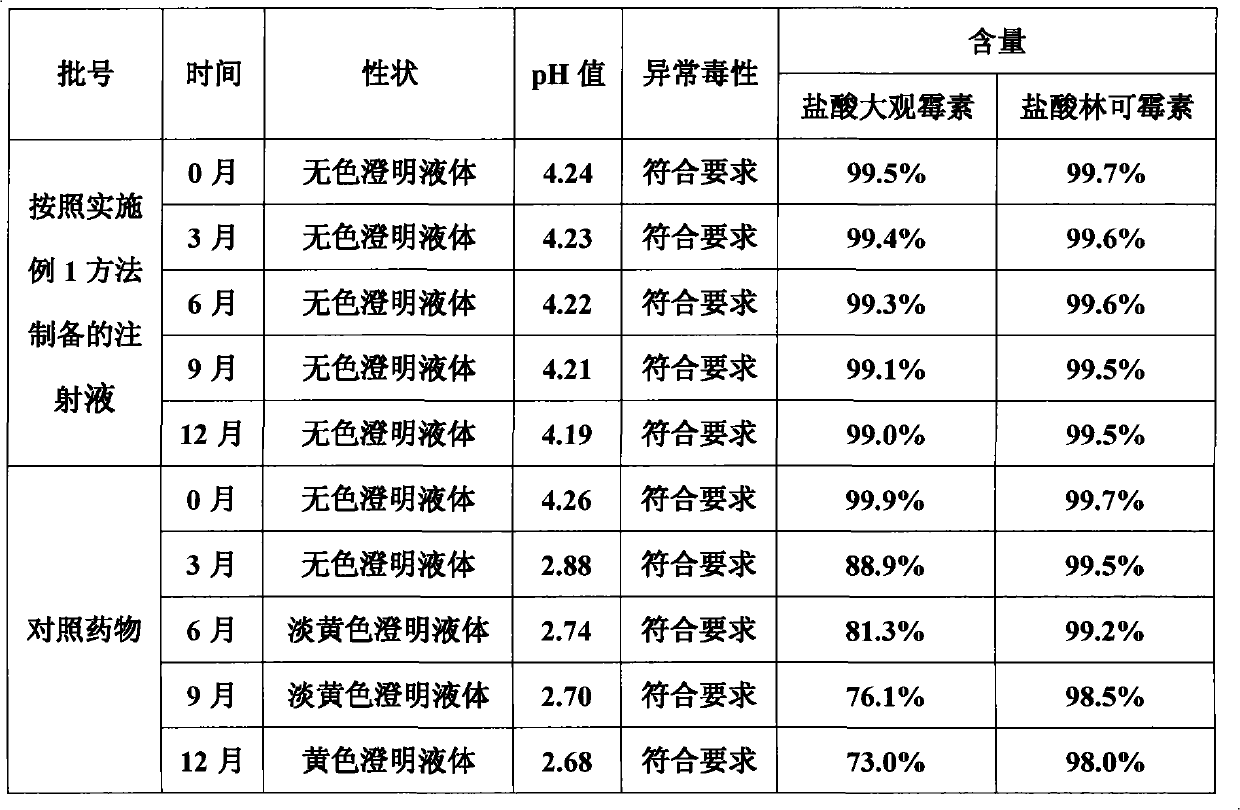
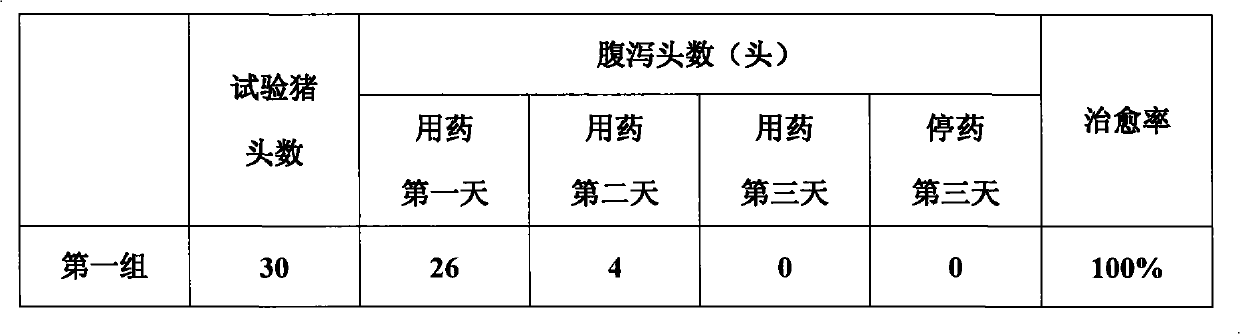
Method for preparing veterinary injection of spectinomycin hydrochloride and lincomycin hydrochloride
A technology of spectinomycin hydrochloride and lincomycin hydrochloride, which is applied to medical preparations containing active ingredients, devices for making medicines into special physical or ingestible forms, and pharmaceutical formulations, etc. Meet the quality standards, can not get spectinomycin hydrochloride, lincomycin hydrochloride injection and other problems, to achieve the effect of simple steps, stable quality and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] prescription:
[0032] Spectinomycin Hydrochloride 10g
[0033] Lincomycin Hydrochloride 5g
[0034] Hydroxypropyl-β-cyclodextrin 5g
[0035] Antioxidant amount
[0036] Add water for injection to 100mL
[0037] Preparation:
[0038] (1) Take 70mL of water for injection, add hydroxypropyl-β-cyclodextrin according to the weight ratio, add spectinomycin hydrochloride, and stir rapidly at 50°C for 30 minutes;
[0039] (2) Add lincomycin hydrochloride, stir and dissolve;
[0040] (3) Take a small amount of water for injection, add antioxidant, stir to dissolve;
[0041] (4) Merge (2), (3) liquid, make to volume with water for injection;
[0042] (5) Filtration, potting, and microporous membrane filtration to sterilize.
Embodiment 2
[0044] prescription:
[0045] Spectinomycin Hydrochloride 4g
[0046] Lincomycin Hydrochloride 2g
[0047] Hydroxyethyl-β-cyclodextrin 40g
[0048] Antioxidant amount
[0049] Add water for injection to 100mL
[0050] Preparation:
[0051] (1) Prepare hydroxyethyl-β-cyclodextrin cyclodextrin into a saturated solution at 50°C, add spectinomycin hydrochloride according to the weight ratio, stir for 30 minutes, rapidly cool down to below 4°C, filter, and dry to obtain hydrochloric acid Spectinomycin inclusion complex;
[0052] (2) Take 70 mL of water for injection, add spectinomycin hydrochloride inclusion compound, stir to dissolve, add lincomycin hydrochloride and stir to dissolve;
[0053] (3) Take a small amount of water for injection, add antioxidant, stir to dissolve;
[0054] (4) Merge (2), (3) liquid, make to volume with water for injection;
[0055] (5) Filtration, potting, drilling 60 ray sterilization.
Embodiment 3
[0057] prescription:
[0058] Spectinomycin Hydrochloride 30g
[0059] Lincomycin Hydrochloride 15g
[0060] γ-cyclodextrin 1g
[0061] Antioxidant amount
[0062] Add water for injection to 100mL
[0063] Preparation:
[0064] (1) Take 70mL of water for injection, add γ-cyclodextrin according to the weight ratio, add spectinomycin hydrochloride, and stir rapidly at 60°C for 30 minutes;
[0065](2) Add lincomycin hydrochloride, stir and dissolve;
[0066] (3) Take a small amount of water for injection, add antioxidant, stir to dissolve;
[0067] (4) Merge (2), (3) liquid, make to volume with water for injection;
[0068] (5) Filtration, potting, and microporous membrane filtration to sterilize.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com