New compounds as adenosine a1 receptor antagonists
The technology of a compound, -R1, is applied in the field of new compounds as adenosine A1 receptor antagonists, which can solve the problems of limited potency and selectivity disclosed
Active Publication Date: 2010-09-15
PALOBIOFARMA SL
View PDF1 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
A report (Ijzerman P, et al., J. Med. Chem. 2001, 44, 749-762) describes the selective A 1 Antagonist, but disclosed limited potency and selectivity, and results were obtained using rat adenosine receptors instead of human adenosine receptors
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
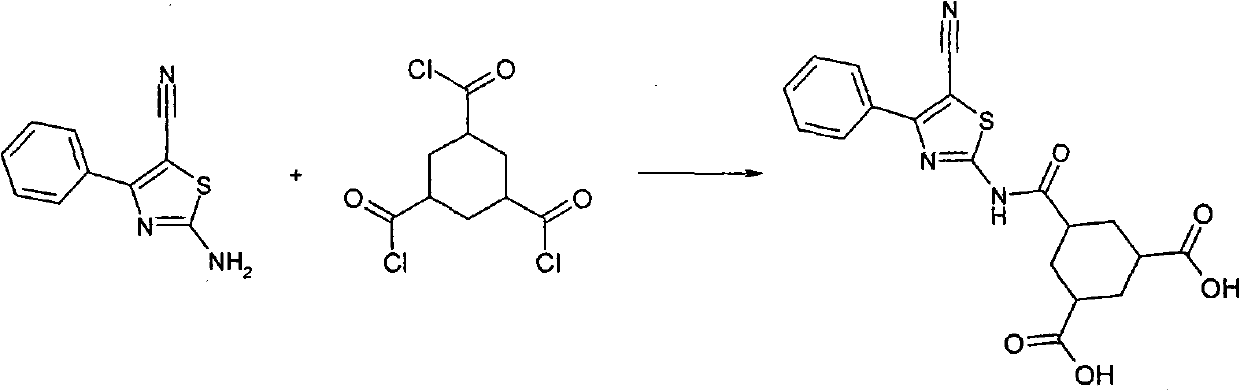
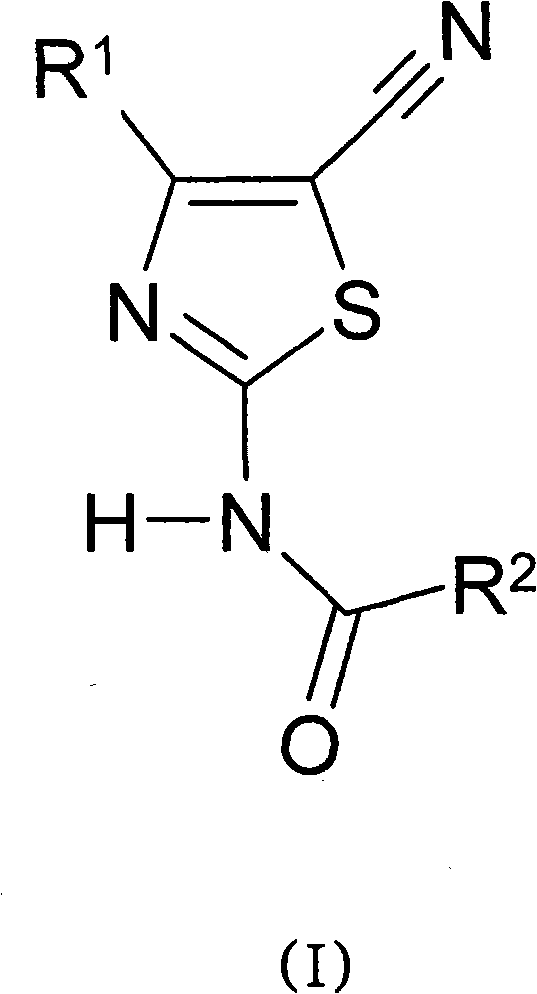
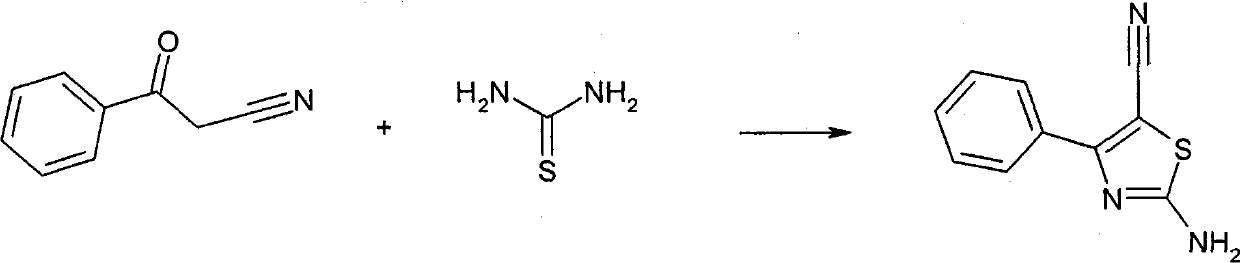
This compounds correspond to the formula (I), where: R1 represents and aryl or heteroaryl group optionally substituted by one or more substituents selected from the group consisting of halogen atoms, straight or branched optionally substituted lower alkyl, cycloalkyl, hydroxy, straight or branched, optionally substituted lower alkoxy, cyano, or -CO2R', wherein R' represents a hydrogen atom or a straight or branched, optionally substituted lower alkyl group; R2 represents a group selected from: a) a straight or branched lower alkyl group substituted by one or more carboxylic groups (-COOH) andoptionally substituted by one or more halogen atoms; b) a cycloalkyl group substituted by one or more carboxylic groups (-COOH) and optionally substituted by one or more halogen atoms; c) a straight or branched alkylcycloalkyl or cycloalkylalkyl group substituted by one or more carboxylic groups (-COOH) and optionally substituted by one or more halogen atoms.
Description
field of invention The present invention relates to novel antagonists of adenosine receptors, in particular A 1 Antagonists of adenosine receptor subtypes, relating to the use of said compounds in the treatment of diseases susceptible to amelioration by antagonism of adenosine receptors, in particular in the treatment of cardiovascular, renal and respiratory diseases , these diseases are known by using A 1 Antagonists of adenosine receptors are improved, more specifically diseases such as congestive heart failure, renal failure, hypertension, hypotension during dialysis, ischemia, supraventricular arrhythmias, myocardial reperfusion injury, asthma, COPD and Allergic rhinitis, also relates to pharmaceutical composition comprising said compound. Background of the invention The effects of adenosine are via at least four receptors hitherto known as belonging to the G protein-coupled receptor family A 1 、A 2A 、A 2B and A 3 It is mediated by specific cell membrane receptors ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D277/56C07D417/04A61K31/426A61P9/00
CPCC07D277/56C07D417/04A61P11/02A61P11/06A61P11/08A61P13/12A61P17/00A61P17/04A61P19/02A61P25/22A61P25/28A61P37/08A61P43/00A61P9/00A61P9/06A61P9/10A61P9/12A61K31/426
Inventor L·冈扎雷兹利奥J·A·卡马乔高梅兹
Owner PALOBIOFARMA SL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com