Pharmaceutical compositions containing the enzyme cyprosin, an aspartic peptidase from cynara cardunculus and its inclusion in antitumour formulations
A composition and technology of cardoon, applied in the field of recombinant cyprosin and pharmaceutical preparations, can solve the problem of no direct relationship report between peptidase and PCD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
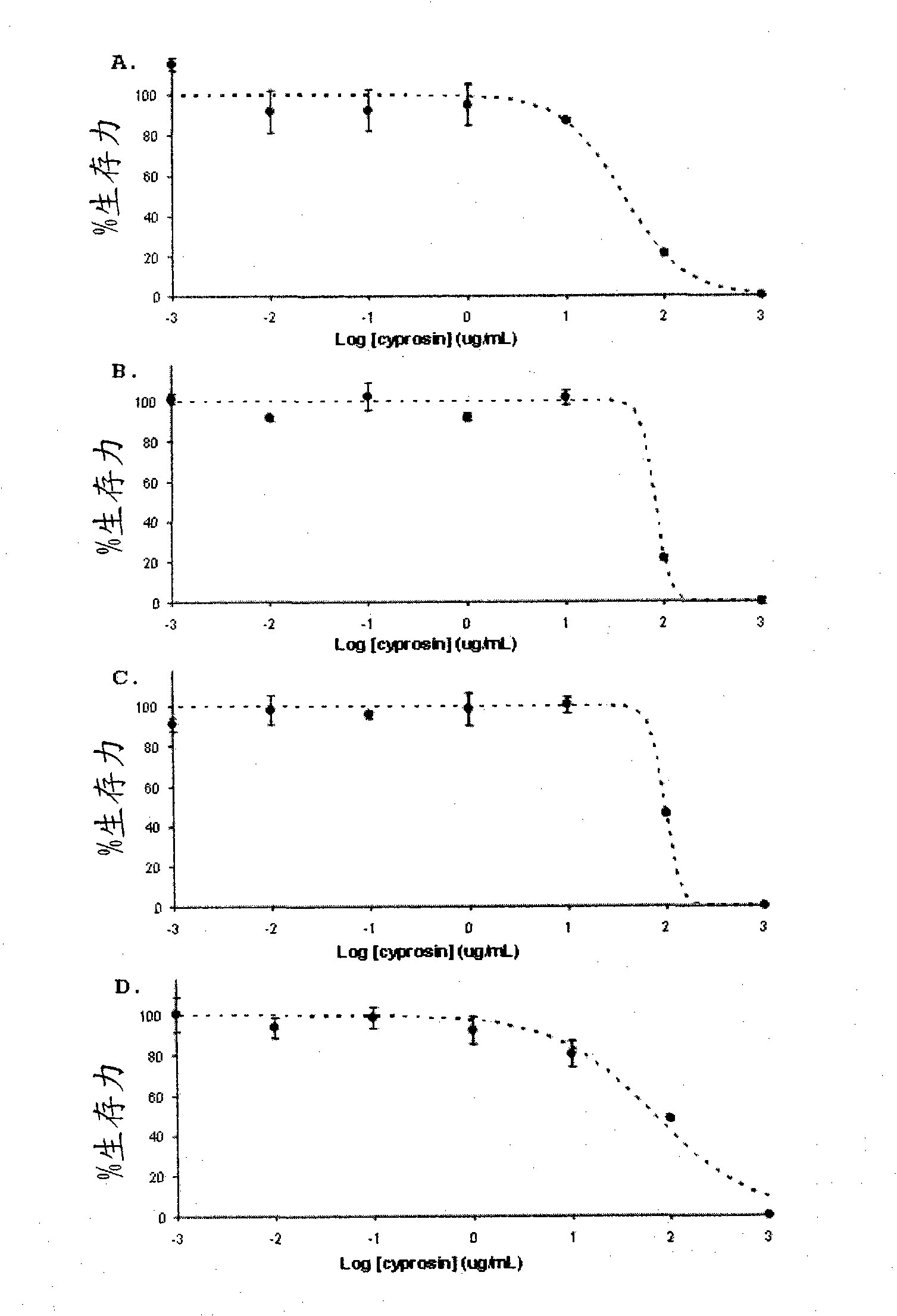
[0049] Antitumor activity of natural cyprosin preparations comprising two structural chains: N-terminal chain (composed of N-terminal propeptide and mature N-terminal) and C-terminal chain (mature peptide C-terminal) , which is isolated and purified from dried cardoon flowers.
[0050] The cyprosin preparation was obtained from dried cardoon flowers as previously described by Brodelius et al., 1995 . The antitumor activity of the enzyme preparation was evaluated with 4 kinds of human tumor cell lines and 2 kinds of non-tumor cell lines. The 4 kinds of human tumor cell lines were: epithelial cell lines derived from colon cancer (HCT116, ATCC CCL-247), Epithelial cell line from fibrosarcoma (HT1080, ATCCCCL-121), rhabdomyosarcoma (TE671, ATCC CCL-136), and adenocarcinoma (Hela, ATCC CCL-2TM) ; The 2 non-neoplastic cell lines: one consisting of human intestinal (epithelial) cells (FHs74Int, ATCC CCL-241) and the other consisting of Vero cells (Vero, ATCC CRL-1587).
[0051] The...
Embodiment II
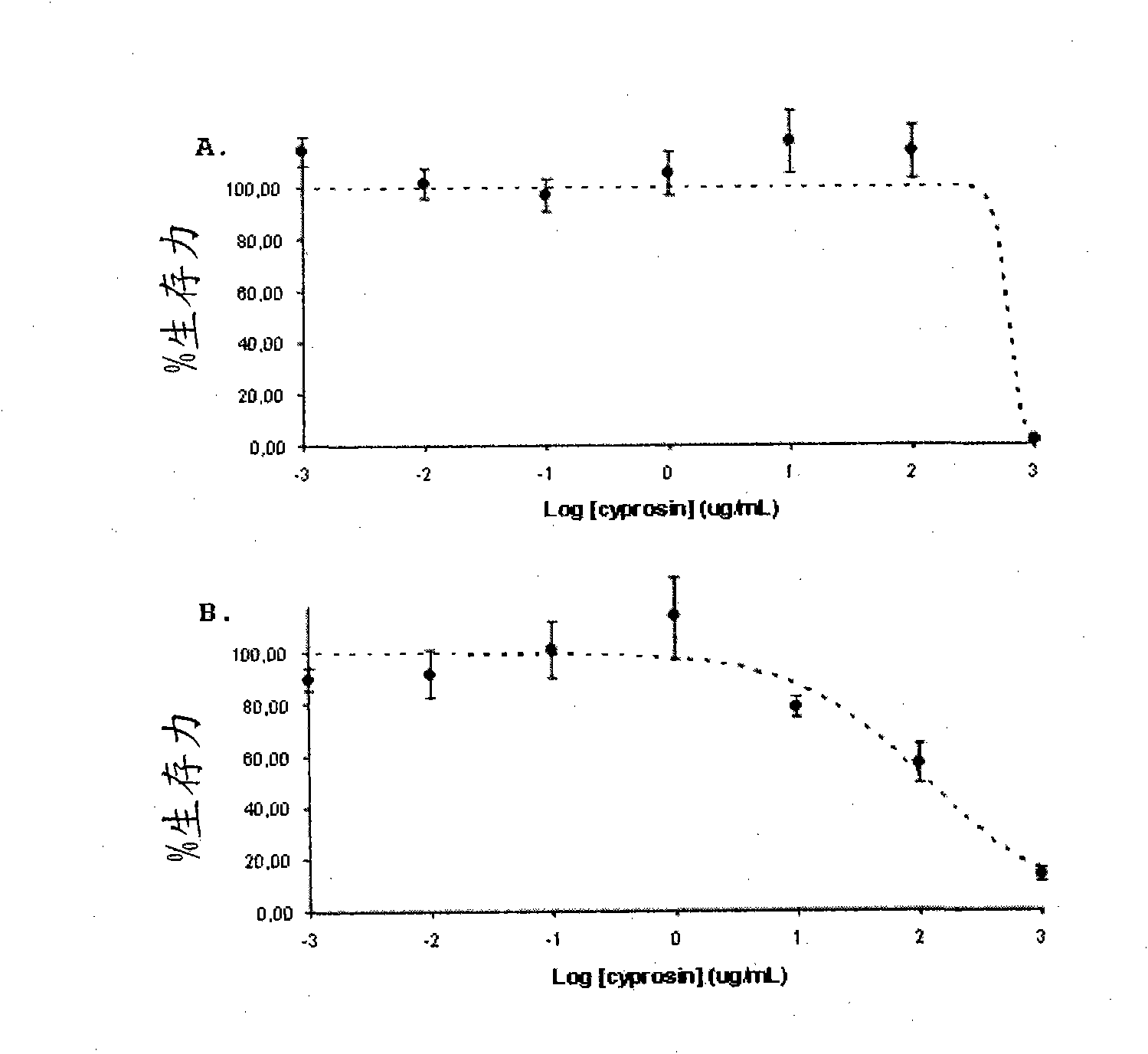
[0096] Antitumor activity of recombinant cyprosin preparations comprising two structural chains: the N-terminal chain (composed of the N-terminal propeptide and the mature N-terminal) and the C-terminal chain (composed of the mature C-terminal peptide ) was isolated and purified from the culture medium of Saccharomyces cerevisiae strain transformed with CYPRO11.
[0097] The cyprosin preparation was obtained from the culture supernatant of a Saccharomyces cerevisiae strain (BJ1991 ) transformed with the CYPRO11 gene encoding cyprosin as described above (Pais et al., 2000). The antitumor activity of the enzyme preparation was determined on a human tumor epithelial cell line derived from colon cancer (HCT116, ATCCCCL-247) and a non-tumor cell line composed of human intestinal epithelial cells (FHs74Int, ATCC CCL-241).
[0098] The tumor cell line HCT116 was inoculated on basal medium DMEM (Cambrex) supplemented with fetal bovine serum (FBS-Gibco). The final concentrations of gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com