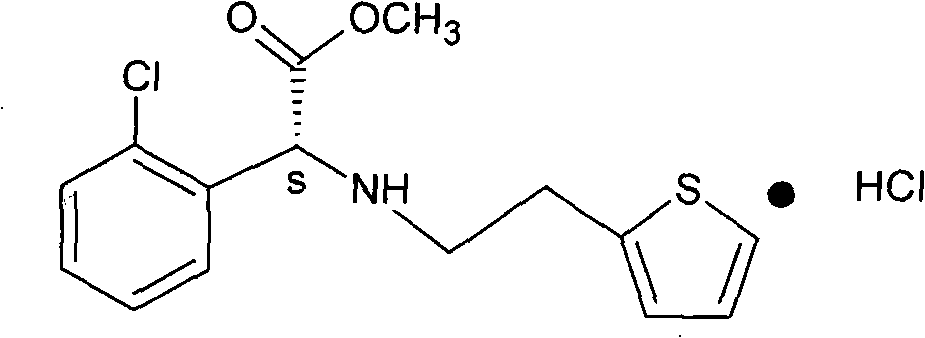
Anti-racemization preparation method of D-(+)-alpha-(2-thienylethamino)-alpha-(2-chlorophenyl) acetate hydrochloride
A technology of thienylethylamine and o-chlorophenylglycine methyl ester, which is applied in the field of preparation of D--α--α-methyl acetate hydrochloride, to achieve the effect of reducing the degree of racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 60 grams of refined dry product of S-o-chlorophenylglycine methyl ester-L-tartrate, 180 ml of chloroform, and 60 grams of water were put into a 500 ml reactor. Cool to below 10°C. Add ammonia water dropwise at 10°C to adjust the pH to 8-9, stir for 0.5 hours, let stand for 30 minutes, separate layers, extract the water layer with 50ml of chloroform, combine the chloroform layers, wash with 100ml of water, and drain the water layer. The chloroform layer was concentrated under reduced pressure to 55-60°C, and the vacuum degree was -0.095~-0.090Mpa to obtain about 34g of S-o-chlorophenylglycine methyl ester as a light yellow oily product, with a yield of 99%.
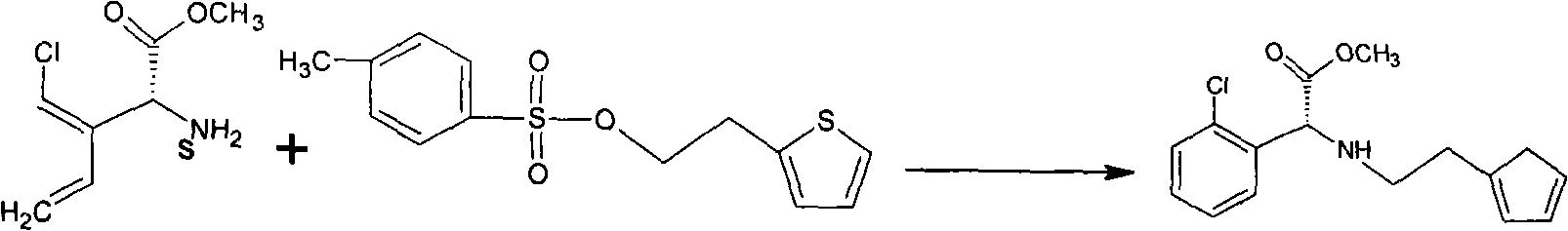
[0015] 180 grams of the above-mentioned S-o-chlorophenylglycine methyl ester, dehydrated and deacidified acetonitrile (acetic acid content less than 0.01%, moisture less than 0.05%), 60 grams of sulfonate, and 40 grams of dipotassium hydrogen phosphate were added in a 500ml reactor. After the addition is complete, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com