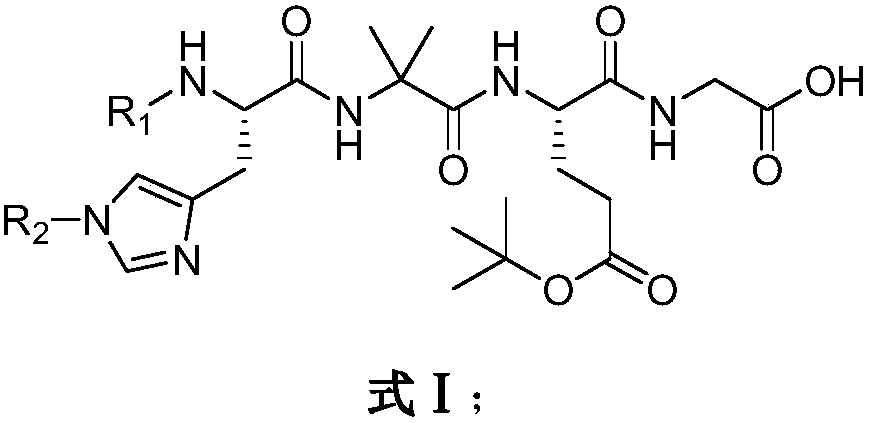
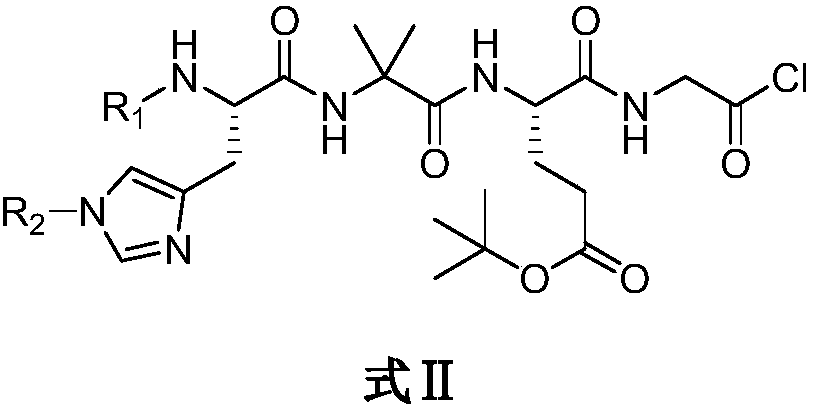
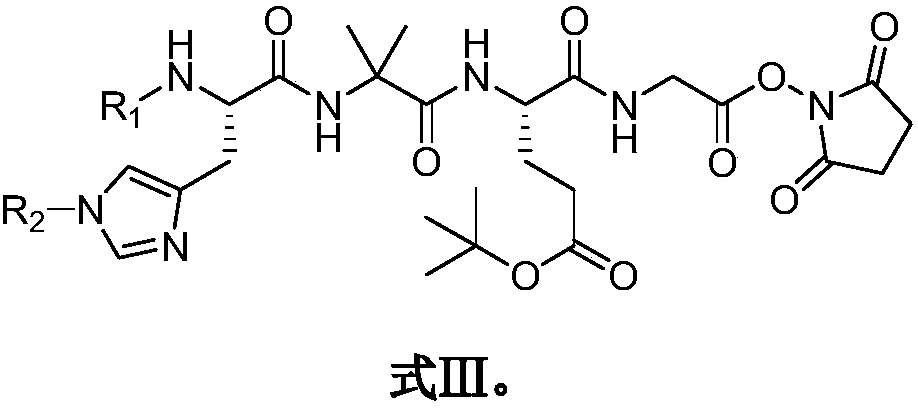
New polypeptide fragment of somalutide and preparation method thereof
A peptide fragment and fragment technology, applied in the field of peptide drug synthesis, can solve the problems of reduced coupling efficiency, increased amino acid racemization, decreased product purity and yield, etc., to improve coupling efficiency, reduce the degree of racemization, and improve product quality. quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the preparation of Fmoc-His(Boc)-Aib-Glu(OtBu)-Gly-OH
[0040] Dissolve Cbz-Glu(OtBu)-OH (67.5g, 200mmol) and glycine methyl ester hydrochloride (25.1g, 200mmol) in 550ml THF, add dicyclohexylcarbodiimide (45.4g, 220mmol) and HOBt (40.5g, 300mmol), stirred at room temperature for 18 hours. After the reaction, filter the reaction solution, wash the filter cake with tetrahydrofuran, combine the filtrates, concentrate under reduced pressure, and then re-dissolve in dichloromethane, leave the solution at 0°C for 30 minutes, then filter, and wash the solid with dichloromethane , concentrated under pressure after the filtrate was combined to obtain Cbz-Glu(OtBu)-Gly-OMe (76.6g, 93.8%), Ms=408.19 (M+H + ).
[0041] Cbz-Glu(OtBu)-Gly-OMe (75g, 183.6mmol) was dissolved in 600ml of dichloromethane containing 11.4ml of HCl, nitrogen was evacuated and Pd / C catalyst was added, followed by hydrogen for reduction reaction. After the reaction is finished, the catalyst ...
Embodiment 2
[0045] Embodiment 2, the preparation of Boc-His-Aib-Glu(OtBu)-Gly-OSu
[0046] Cbz-Glu(OtBu)-OH (50.6g, 150mmol) and glycine methyl ester (18.8g, 150mmol) were dissolved in 500ml tetrahydrofuran, and dicyclohexylcarbodiimide (34g, 165mmol) and HOBt (30.4g, 225mmol), stirred at room temperature for 18 hours. After the reaction, filter the reaction solution, wash the filter cake with tetrahydrofuran, combine the filtrates, concentrate under reduced pressure, and then re-dissolve in dichloromethane, leave the solution at 0°C for 30 minutes, then filter, and wash the solid with dichloromethane , combined the filtrates and concentrated under pressure to obtain Cbz-Glu(OtBu)-Gly-OMe (57.2 g, 93.3%). Ms=408.19(M+H + ).
[0047] Cbz-Glu(OtBu)-Gly-OMe (56g, 137.1mmol) was dissolved in 500ml of dichloromethane containing 8.5ml of HCl, nitrogen was evacuated and Pd / C catalyst was added, followed by hydrogen for reduction reaction. After the reaction is finished, the catalyst is remov...
Embodiment 3
[0052] Embodiment 3, the preparation of Fmoc-His(Fmoc)-Aib-Glu(OtBu)-Gly-OH
[0053] Weigh Cbz-Glu(OtBu)-OH (16.9g, 50mmol) and glycine methyl ester (6.3g, 50mmol) and dissolve in 150ml tetrahydrofuran, add dicyclohexylcarbodiimide (11.4g, 55mmol) and HOBt (10.1 g, 75mmol), stirred at room temperature for 18 hours. After the reaction, filter the reaction solution, wash the filter cake with tetrahydrofuran, combine the filtrates, concentrate under reduced pressure, and then re-dissolve in dichloromethane, leave the solution at 0°C for 30 minutes, then filter, and wash the solid with dichloromethane , combined the filtrates and concentrated under pressure to obtain Cbz-Glu(OtBu)-Gly-OMe (18.9 g, 92.4%). Ms=408.19(M+H + ).
[0054] Cbz-Glu(OtBu)-Gly-OMe (16g, 39.2mmol) was dissolved in 150ml of dichloromethane containing 2.4ml of HCl, nitrogen was evacuated and Pd / C catalyst was added, followed by hydrogen for reduction reaction. After the reaction is finished, the catalyst i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com