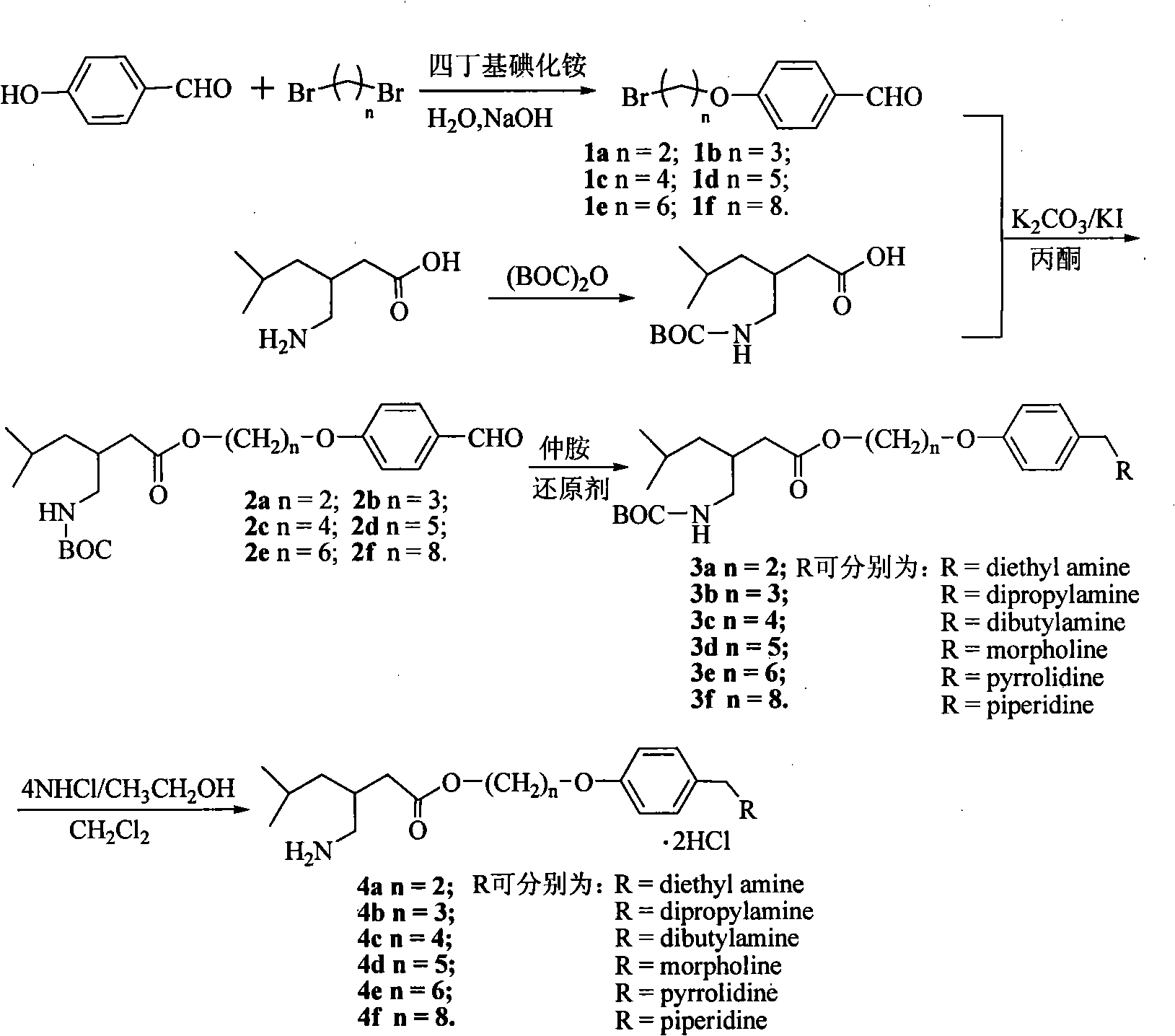
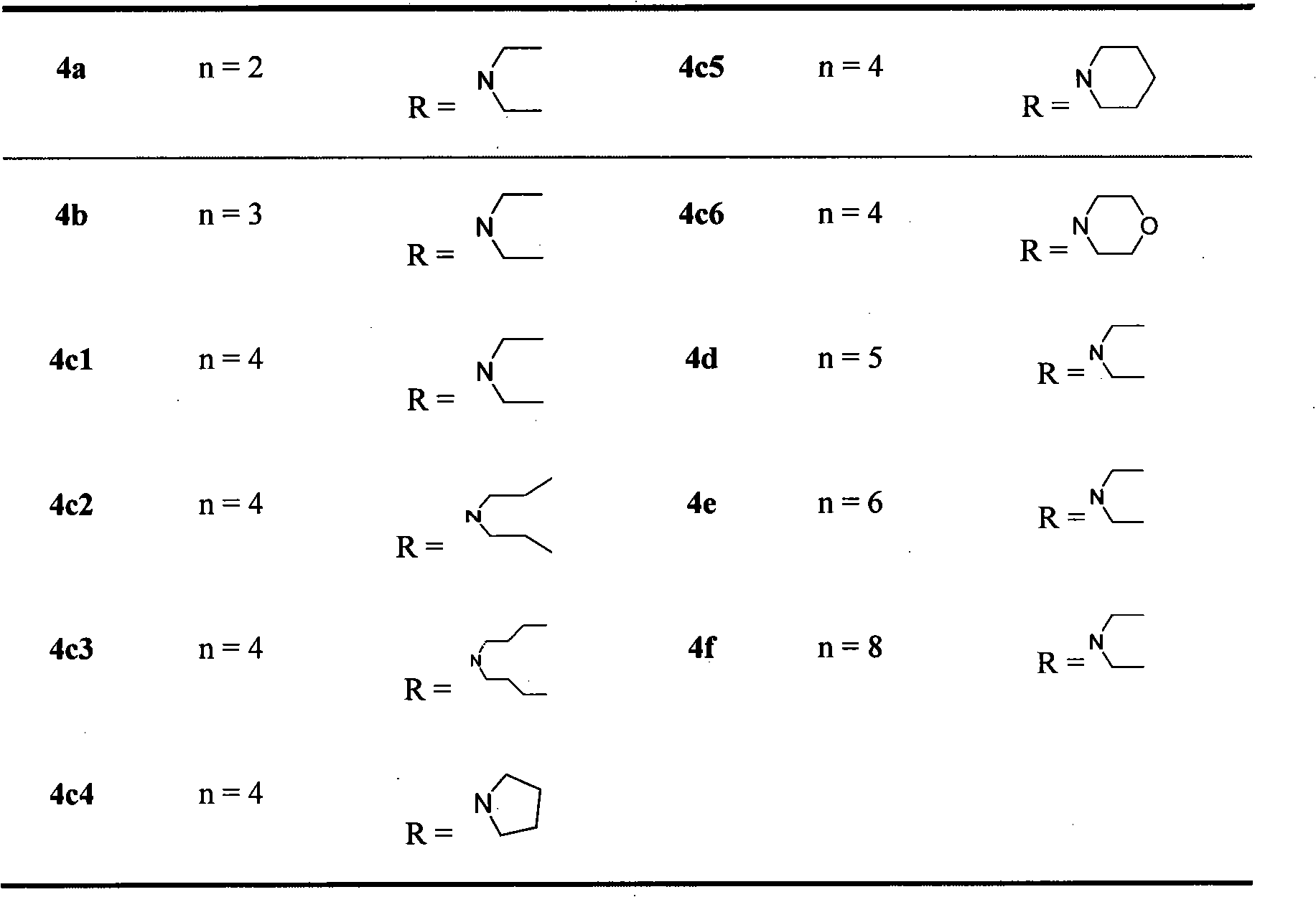
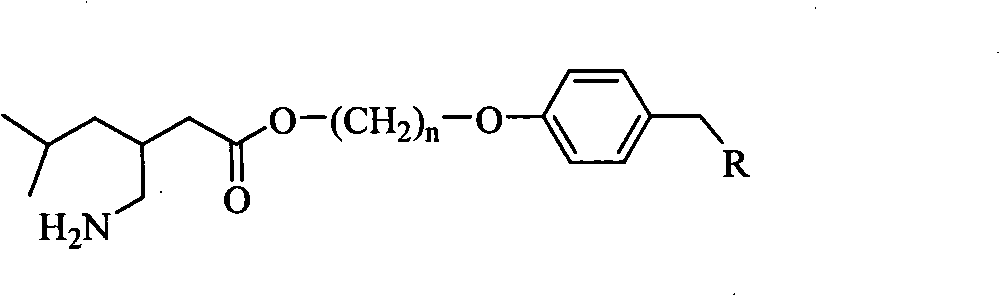Pregabalin derivative and application thereof
A technology of pregabalin and derivatives, applied in new pregabalin derivatives and their application fields, can solve problems such as limitation and wide application of side effects, and achieve obvious selectivity and significant resistance to acetylcholinesterase and butyrylcholine Effect of Esterase Activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment one: the synthesis of 4-(8-bromooctyloxy)benzaldehyde (1f)
[0023] First add 2.4g (20mmol) p-hydroxybenzaldehyde, 15ml 1.8-dibromooctane and 5.52g (40mmol) potassium carbonate (microwave activation) into a 100ml round bottom flask, add 40ml of anhydrous acetone, and stir under reflux 24 hours. TLC tracked until the reaction was complete. Filter and spin dry the solvent to obtain the initial product. The yellow solid powder 1f was separated by silica gel column chromatography, and the yield was 63%. That 1 HNMR (CDCl 3 , 300MHz) δ, ppm data are: 9.86(s, 1H, CHO), 7.82(d, J=8.8Hz, 2H, ArH), 6.99(d, J=8.7Hz, 2H, ArH), 4.04(t, J=6.5Hz, 2H, H1), 3.20(t, J=7.0Hz, 2H, H8), 1.88-1.78(m, 4H, H7, H2), 1.42-1.30(m, 8H, H3, H4, H5 , H6). The structure of the product was thus determined.
[0024] 1a, 1b, 1c, 1d, 1e were prepared by the same method as above.
Embodiment 2
[0025] Embodiment two: BOC protects the synthesis of pregabalin p-n-octyloxybenzaldehyde ester (2f)
[0026] Take 2mmol of 4-(8-bromooctyloxy)benzaldehyde (1e) and 20ml of dry acetone in a 50ml flask, add 1.2g (5mmol) of KI, and stir overnight at room temperature. Then 0.54g (2mmol) of BOC-protected pregabalin and 1g (7.5mmol) of microwave-activated K 2 CO 3 Add the reaction system, heat up to 60°C and reflux for 24 hours. After treatment, potassium carbonate was removed by filtration, and the solvent was evaporated under reduced pressure. The crude product was subjected to silica gel column chromatography (developing solvent: 1:9 ethyl acetate:petroleum ether-1:2 ethyl acetate:petroleum ether gradient elution) to obtain BOC Protection of pregabalin p-n-octyloxybenzaldehyde ester (2f). product structure 1 HNMR analysis data confirmed.
[0027] 1 H NMR (CDCl 3 , 300MHz) δ, ppm: 9.86(s, 1H, CHO), 7.82(d, J=8.6Hz, 2H, ArH), 6.99(d, J=8.6Hz, 2H, ArH), 3.92(t, J= 6.2Hz, 2H,...
Embodiment 3
[0029] Example 3: Synthesis of BOC-protected pregabalin p-ethoxybenzyldiethylamino ester (3a).
[0030] Take 1 mmol of BOC-protected pregabalin p-n-butoxybenzaldehyde ester (2a), dissolve it in 20 ml of dry 1,2-dichloroethane, add 0.1 ml (1 mmol) of secondary amine and stir for 5 minutes. Then 0.7 g of sodium triacetoxyborohydride was added and stirred overnight. After the reaction was complete as detected by TLC, a saturated sodium bicarbonate solution was added to terminate the reaction, and extracted three times with ethyl acetate. The combined organic phases were dried. After the solvent was spin-dried, the crude product was subjected to silica gel column chromatography (developing solvent: 1:50 methanol:dichloromethane) to obtain BOC-protected pregabalin p-n-butoxybenzyldiethylamino ester (3a) as yellow oil. product structure 1 HNMR analysis data confirmed.
[0031] 1 H NMR (CDCl 3 , 300MHz) δ, ppm: 7.22 (d, J=8.6Hz, 2H, ArH), 6.84 (d, J=8.6Hz, 2H, ArH), 4.42-4.35 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com